Introduction
Diseases associated with the Central Nervous System (CNS) represent a group of conditions with significant social and economic repercussions. They are characterized by a high prevalence in both developed and developing countries. Cerebrovascular diseases (CVD) and those caused by neuronal degeneration attract the attention of major research centers. Nowadays, there is no effective treatment on the market that achieves a cure for these diseases.1,2
Currently, there is a lot of research on the neurobiological mechanisms related to brain aging and associated diseases. Scientists continue to search for methods to improve their treatment, both clinical and psychosocial.3,4
Despite the great effort undertaken in the analysis of the pathophysiology of neurological conditions and the development of new drugs to treat these conditions, the success obtained is limited.1,2,4 A growing line of research in neuroscience is the use of human recombinant molecules. Recombinant human erythropoietin with low sialic acid content (NeuroEPO) is a relevant example of the use of these molecules.1,2,3,4 NeuroEPO has cytoprotective, hypoglycemic and neuroprotective effect.4,5,6,7)
The systemic administration of NeuroEPO has the disadvantage that it is quickly degradable at the hepatic level due to the lower amount of sialic acid.1,2,3 That is why different routes of administration have been explored. Intranasal administration would prevent NeuroEPO liver clearance.
Recent studies have shown that intranasal administration of NeuroEPO has a neuroprotective effect on different biomodels of neurological diseases.1,2,3,4,8 This neuroprotective effect has also been demonstrated in controlled clinical trials in Parkinson's and Alzheimer's disease.9,10
However, it is not known whether its intranasal application at maintained doses, for a longer period, could cause alterations in the histological structure of the respiratory mucosa in rats.
The objective of this research is to determine the effect of intranasal administration of NeuroEPO on the histological structure of the respiratory mucosa and its associated lymphatic tissue in Wistar rats.
Materials and Methods
An experimental, longitudinal, and prospective study was conducted. The experiment was conducted at the National Center for the Production of Laboratory Animals (CENPALAB) and the Institute of Basic and Pre-Clinical Sciences Victoria de Girón (ICBP-UCMH) between 2016 and 2017.
Wistar rats were used as a biological model, approximately 21 days old, weighing between 150-175 g, nulliparous and non-gravid, from the CENPALAB. The animals were kept in the animal testing area of the Bioterium, with controlled conditions of temperature (19-25 oC) and relative humidity (40-70 %), light-dark cycles 12/12h, and access to water and food ad libitum according to national and international regulations.11 The experiment was carried out with the approval of the Institutional Committee for the Care and Use of Laboratory Animals (CICUAL) of CENPALAB.
NeuroEPO Formulation
The nasal formulation of NeuroEPO (PATENTS PCT / cu2006 / 000001 and 20050138 to CIDEM, Havana, Cuba)12 was prepared by the Center for Drug Research and Development (CIDEM) and supplied by the Center for Molecular Immunology (CIM) through the marketer of biopharmaceutical products of this center (Havana, Cuba).
The IFA (Active Pharmaceutical Ingredient) of this formulation is rHu-EPO with low sialic acid content; it also contains the bioadhesive polymer D-hydroxy-propylcellulose to increase the residence time in the nasal cavity and decrease its elimination by ciliary movement. This was diluted in phosphate-buffered saline (pH 7,0) at 0,15 mM. The nasal formulation also contains other excipients for the stability of the formulation: benzalkonium chloride as a preservative to avoid microbiological contamination to regulate osmotic pressure.12 The vehicle was supplied by the Center for Drug Research and Development (CIDEM) and contained all substances (excipients) except NeuroEPO.
A total of ten healthy animals were randomly distributed in two groups of five animals each, as follows:
Control group, which were treated with vehicle solution intranasally.
Treated group, which was administered NeuroEPO 300 μg / Kg, volume (10 μL) intranasally.
Intranasal application (IN)
Rats in both groups underwent intranasal (IN) treatment. The animal was restrained, placed in supine position and the formulation was administered in each nostril slowly using an automatic pipette; the approximate application time was one or two minutes. The treatment was performed for 28 days, at that day euthanasia was performed.
The control group was given a vehicle at a rate of 0,3μl/g. The treated group received 300 μg/kg/day from NeuroEPO. This dose was obtained from the human dose of recombinant EPO of 48 μg/kg/day or 1000 IU/kg/day, which is twice the dose used to stimulate erythropoiesis with rHu-EPO.9,13
Processing and staining of nasal structures for histological study
The nasal structures were removed, washed with 0,9 % sodium chloride solution and fixed in neutral 4 % formalin. They were then decalcified with formic acid for 14 days. Tissue samples for histopathological examination were obtained from the third level (T3) of the nasal cavity; the palatine structures (through the second palatine ridge and includes the first upper molar) were taken as a reference.14,15 The samples were processed by the conventional method of inclusion in paraffin.16,17
Four slides were obtained per animal, with three cuts of five μm thickness each, obtained in a Histo-Line Laboratories MR 300 microtome with steel blades. The staining techniques used were hematoxylin and eosin (three slides), and PAS / Alcián Blue (one slide).
Qualitative histological study
The slides were observed under a Motic BA 210 Digital optical microscope at 100x, 400x, and 1000x in search for morphological changes in both the epithelium and the lamina propria of the respiratory mucosa. In each cut, ten fields per animal randomly chosen were observed. A general assessment of the entire cut was made, aspects related to the shape, size, coloration, and arrangement of the structures were analyzed; in addition, the presence of possible inflammatory or degenerative changes was considered.18
Morphometric study of the respiratory mucosa
The quantitative study of the respiratory mucosa was carried out. Epithelium height was determined from the basement membrane to the apical surface not including cilia. In the same way, the thickness of the lamina propria was determined.
The morphometric study of the respiratory mucosa was analyzed at the level of the maxillary sinuses, nasopharyngeal duct and ventral portion of the middle septum. For the digitization of the images, a Motic BA 210 Digital microscope was used, with 10x, 40x and 100x lens, Widelfield binocular 300 (F.N.20) Light distribution 100 projection tube and a high-resolution Moticam model digital camera attached to the microscope, equipped with data analysis software with USB 2.0 input to a computer. For the morphometric analysis, the Image Tool Software 3.0 for Windows19 was used.
Statistical analysis
The values of the variables were collected in the database; the Microsoft Excel program was used. The results of the variables were processed using the statistical software Grafpad Prism version 5.01 for Windows.20 The mean and standard deviation were determined as descriptive statisticians. Normality tests were performed on all data (Kolmogorov-Smirnov test). As these did not show normal distribution, the Mann-Whitney test was used to compare the means. In all cases the differences were considered significant for a p< 0,05.
The datasets generated and/or analyzed during the current study are available in the Mendeley Data repository.21
Results
Histological features of the respiratory mucosa
In the respiratory mucosa of the group treated with NeuroEPO, the presence of inflammatory infiltration and signs of fibrosis were not evident. The epithelium of cylindrical ciliated pseudostratified lining with goblet cells in all its extension showed a normal morphological pattern. Mucus-secreting goblet cells were observed with normal histological characteristics. In the underlying lamina propria, abundant blood vessels and seromucosal glands with well-constituted acines of normal morphological appearance were evident. These results correspond to what was shown in the control group. (Figure 1).
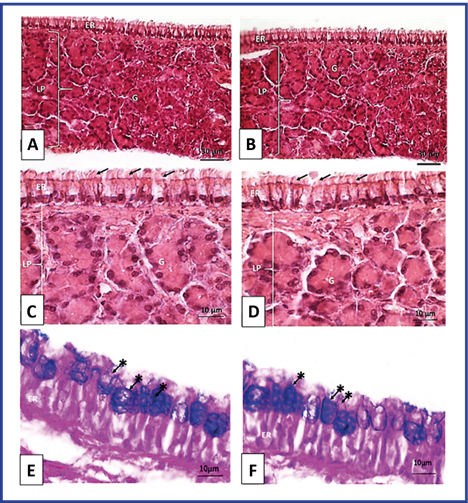
Fig. 1 Optical photomicrography of the respiratory mucosa of Wistar rats. T3 Maxillary sinus. A, C and E: Control group. B, D and F: Group treated with NeuroEPO. ER: respiratory epithelium, LP: self-contained payroll, Arrows: cilia, G: glands. Staining with H/E. A and B magnification 400X. C and D magnification 1000X. PAS/Blue Coloration of Alcián. E and F Magnification 1000X. Arrows with asterisks: goblet cells.
Morphometric Study
Height of the respiratory epithelium and thickness of the lamina propria
The height of the respiratory epithelium, and the thickness of the lamina propria of the respiratory mucosa of animals treated with NeuroEPO were similar to the control. (Figure 2).

Fig 2 Height of the epithelium and thickness of the lamina propria of the respiratory mucosa of Wistar rats. Groups control and treated with NeuroEPO. Significance level p >0.05.
Histological features of lymphoepithelium and nasal mucosal associated lymphatic tissue (NALT)
The histological features observed in the lymphoepithelium and NALT of rats in this group were normal. The lymphoepithelium lining the aggregates of lymphatic follicles showed typical morphological features. The lymphatic follicles showed shape and size similar to those of the control group. Lymphocytes of normal morphological aspect were observed. (Figure 3).
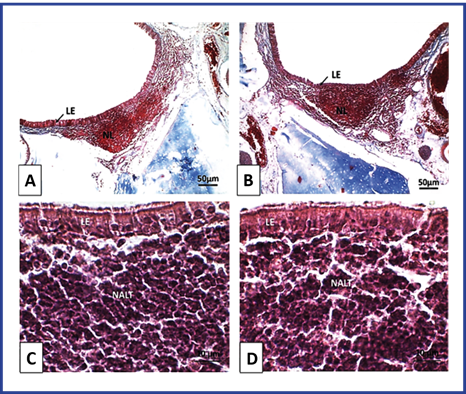
Fig. 3 Optical photomicrography of lymphoepithelium and NALT of Wistar rats. T3. Mucosa of the lateral wall of the anterior orifice of the nasopharyngeal duct). A and C: Control group. B and D: Group treated with NeuroEPO. LE: lymphoepithelium, NL: lymph nodes. NALT: lymphatic tissue associated with the nasal mucosa. A and B Coloration with Mallory's Trichromics. Magnification 100X. C and D Staining with H/E. Magnification 1000X.
Discussion
In the present research, no changes were observed in the histological characteristics of the epithelium or the lamina propria of the respiratory mucosa. Likewise, no signs indicative of acute or chronic inflammatory response were evidenced with intranasal administration of NeuroEPO to Wistar rats. The control and treated groups exhibited similar morphological characteristics.
There are no previous studies describing the effect of intranasal administration of NeuroEPO in the structure of respiratory mucosa. In studies with other molecules as chlorine gas, ozone, formaldehyde, and xylene, exposure causes cilia loss, caliciform cell metaplasia, squamous cell hyperplasia, degeneration, epithelial necrosis, and inflammatory infiltration. Regenerative epithelial proliferation with or without squamous metaplasia and associated inflammatory response have also been described.22,23,24,25,26
The results of the current study differ from some reports suggesting that prolonged use of some drugs nasally triggers drug rhinitis, characterized by squamous metaplasia of the respiratory epithelium and/or ulceration, increased goblet cells, and thickened and hyalinized basement membrane. The lamina propria of the respiratory mucosa in these cases may exhibit edema or fibrosis with a diffuse infiltrate of lymphocytes, histiocytes and eosinophils. Such is the case with some nasal decongestants and anti-inflammatories, such as oxymetazoline, phenylephrine, mometasone and fluticasone.27,28
Recent studies have evaluated the effect of intranasal administration of NeuroEPO on the nasal and olfactory mucosa in rats (after 14 and 28 days respectively).29,30 Both studies showed that the administration of NeuroEPO did not affect the morphology of the structures explored.
In the morphometric study of the respiratory mucosa, no significant differences were observed in the height of the respiratory epithelium or the thickness of the lamina propria in the study groups. There are no reports in the literature describing the effects of the administration of NeuroEPO on the morphometry of the respiratory mucosa. The results differ from some studies carried out in experimental animals in which a decrease in respiratory epithelium and thickness of the lamina propria corroborated by morphometry has been reported in rats exposed to ethanol and cathelicidin.31,32
It is likely that the results obtained in the present study are related to the fact that the distribution of lesions in the nasal cavity depends on the local dose used and the susceptibility of the tissues to damage. The respiratory epithelium is less susceptible to damage by chemical agents than others of the epithelia of the nasal cavity; in addition, the dose used and the time of exposure to it may have influenced the current results.33
In the present research, the control and treated groups showed a normal morphological pattern of the epithelium and the lamina propria of the respiratory mucosa corroborated with the morphometric study. No signs of acute or chronic inflammation were observed in the study groups; this suggests that NeuroEPO administered nasally at doses of 300 μg/kg/day for 28 days does not produce alterations on the respiratory mucosa in Wistar rats.
In our study, no changes were observed in the histological structure of the lymphoepithelium of rats exposed to high doses of nasal NeuroEPO, behaving similarly to the control group. When observing the histological characteristics of the NALT, it was possible to appreciate that its shape and size were similar in both groups.
No reports were found in the reviewed literature about the effects of NeuroEPO on lymphoepithelium and NALT in rats, which could be compared with those of current research. However, the current results differ from those obtained in other studies, in which it has been reported that animals exposed to toxins nasally triggered alterations in the immune system shortly after their exposure, among these are: decrease in the number of intraepithelial lymphocytes, and lymphocytes present from the lymphatic follicles. These changes had an impact on the shape and size of the NALT of the animals under study.34,35
Rats exposed to high concentrations of inhaled formaldehyde have shown lymphoepithelial hyperplasia. The NALT in these cases showed decreased cellularity of T and B lymphocytes.36
One of the mechanisms through which a toxic agent causes alteration in the immune system is related to the stress it generates on the cells. The sympathetic nervous system is essential to restore homeostasis, altered by stress and to promote the survival of the organism. For each type of aggressor agent, a certain type of specific response is generated.37,38 Stress causes an increase in the secretion of glucocorticoid hormones and catecholamines by activation of the hypothalamic-pituitary-adrenal axis. These hormones interact with receptors on immune cells and cause changes in their response that include modifications in cell traffic and proliferation, changes in cytokine secretion, antibodies, and cytolytic activity.39
Glucocorticoids inhibit myelopoiesis and production of pro-inflammatory cytokines (IL-1, IL-6, TNF). They also induce expression of MHC class II molecules and suppress the function of Th1 lymphocytes.36,37,38 Cortisol is the main glucocorticoid involved in immunomodulation, triggering immunosuppressive and anti-inflammatory effects.37,38,39
One of the factors involved in immunogenicity is the concentration of the drug. A high dose was used in the present study, as reported in the literature.13 However, no effect on lymphoepithelium or NALT was observed. This could be related to a tolerogenic response, and this favored an immunological tolerance.18 This may be related to the fact that the structure of NeuroEPO is very similar to that of endogenous EPO (due to its human recombinant origin).
As a result, NeuroEPO administered nasally at doses of 300 μg/kg/day for 28 days does not produce alterations in lymphoepithelium and NALT in Wistar rats.
This research was limited to the evaluation of the nasal administration of NeruroEPO in the histological structure of the respiratory mucosa and NALT, but further research could be addressed to evaluate the effect in functional aspects.















