Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Ciencias Técnicas Agropecuarias
versão On-line ISSN 2071-0054
Rev Cie Téc Agr vol.23 no.1 San José de las Lajas jan.-mar. 2014
PUNTOS DE VISTA
Suitability of the leaf energy balance model as a topic for designing open_ended problems in physics as a discipline in agriculture profile undergraduate programs
Conveniencia del modelo del balance energético foliar como contenido para el diseño de problemas de física de respuesta abierta para estudiantes de carreras de perfil agropecuario
Dr.C. Juana Dominguez Mora, Dr.C. Luis Raul Parra Serrano, Dr.C. Eduardo Velasco Benitez, Dr.C, Eva Sanchez Garcia
Universidad de Granma, Bayamo, Granma, Cuba.
ABSTRACT
Although commonly considered a topic for Plant Ecophysiology, the leaf energy balance model can be included in Physics courses for undergraduate program adapting its scope in accordance with the learning outcomes suited for the course in which it is applied. The main features of the model are described here and some considerations related to its application at the Universidad de Granma, Cuba, as an element of an instruction program aimed at increase the learner autonomy.
Key words: learner autonomy, solving problems, plant ecophysiology, student creativity.
RESUMEN
Aunque realmente resulta apropiado para un curso de Ecofisiología Vegetal, el modelo del balance energético foliar puede ser adaptado para un curso de Física, teniendo en cuenta los objetivos de aprendizaje de este último. Una propuesta en ese sentido se explica aquí, complementada con algunas consideraciones sobre su aplicación en la Universidad de Granma, en Cuba, como parte de una experiencia encaminada a incrementar la autonomía en el aprendizaje mediante la resolución de problemas de respuesta abierta.
Palabras clave: autonomía del estudiante, resolución de problemas, ecofisiología vegetal, creatividad del estudiante.
INTRODUCCIÓN
The leaf energy balance model (LEBM) has been used for the teaching process of Plan Ecophysiology (Souza, 2003). Nevertheless at the University of Granma, Cuba, it has been used within Physics teaching during several years as part and culminant part of an instruction system intended to promote the autonomous learning (Dominguez et al., 2012). Based on the LEBM, open_ended problems can be designed that, at the same time, operate as context riched problems (Sevrin, 2010; Stinner, 2008; Garrett, 1986). All of that, at the formative level, is suppose that contributes to the autonomous learning through improving the creative capacity of the students (García & García, 2001; Selcuk et al., 2008; Cildir, 2011). Here, the LEBM, as a didactic case, including an example of its use as an open_ended problem is presented and at the same time, some of its features as a students creativity enhancing resource are commented.
The lebm as a didactic case for solving problems in a physics course
Below, the energy balance equation for a leaf is developed, considering that for a leaf at equilibrium, the amount of energy that enters via solar radiation plus ambient heat is equal to that that exits leaf via radiation, heat loss, and transpired water, in other words: energy intothe leaf equals energy out of it. Here, Nobel (2005) and Souza (2010), were considered as essential sources. So, the various contributors to the energy balance of a leaf can be summarized as follows:
Energy into the leaf (absorbed): 1) solar irradiation and 2) infrared radiation (from the surroundings and the sky)
Solar irradiation (S): Solar irradiation can reach a leaf in many different ways, being the direct sunlight (Sdir) the most obvious. Alternatively, the leaf can receive diffuse sunlight (Sdif), coming as scattered light by the molecules and particles in the atmosphere before striking the leaf. Taking into account the leaf inclination (i), i.e. the angle that the leaf plane makes with the horizontal plane, and the absorption properties of the leaf, described by the absorptivity (a), the total absorbed solar irradiation will be:
![]()
The letter S is used for solar irradiation taking into account its spectral feature, that is, its wavelengths are short relative to the earth and sky radiation.
Realistic values for Sdir are between 200 and 800 W·m-2, for a between 0,4 and 0,6; and for sake of simplicity Sdif can be taken as 1000 -Sdir, the leaf inclination can be any between 0° and 90°. Let Sdir, for some condition, equals 600 W·m-2, reaching a leaf characterized by an absorptivityof 0,5; and leaf inclination of 30°;Sdif would equal400 W·m-2, what results in total absorbed irradiation of 460 W·m-2 (rounded).
Infrared radiation from the sky and the surroundings(L): Beside solar irradiation, infrared, or thermal radiation, is also absorbed by a leaf. Any object emits such thermal radiation, including the leaf surroundings (Lsurr) and the sky (Lsly). For considering the amount of thermal radiation absorbed by a leaf, the Stefan_Boltzmann law, which predicts the rate of energy radiation emitted by a blackbody radiator is used, employing the effective temperature, that is, the temperature (should it be for the sky and the surroundings) for that the value (Teff)4 equals the corresponding energy flux. For instance, Tsky is not the temperature we would measure at some particular location in the atmosphere, although σ(Tsky)4 equals the actual amount of radiant energy from above the leaf (sky). By the Stefan_Boltzmann law, with effective temperatures to give the radiation emitted by the surroundings and the sky, the infrared (IR) absorbed by the leaf is:
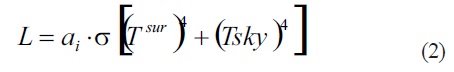
Where the absorptivityai is the fraction of the energy of the incident infrared radiation absorbed by the leaf, and is the well known Stefan_Boltzmann constant, 5,67×10-8 Wm-2K-4. Here it is important to note that the leaf absorptivity in the infrared region of the electromagnetic spectrum differs from that for visible,so, for the leaf absorptivity in the infrared the value of 0,96 will always be used. (For example of computing this term see problem below)
Energy out of leaf: 1) emitted infrared radiation, 2) convection heat, 3) conduction heat and 4) transpiration heat (loss accompanying water evaporation)
Emitted infrared radiation (Le): Thermal radiation is also emitted by the leaf. Such radiation occurs at wavelengths far into the IR because of common leaf temperatures, like those of the surroundings, are near 300 K. This energy flux is expressed following the Stefan_Boltmann law using the leaf temperature (Tleaf). For the general emission case a coefficient known as emissivity(e) is introduced, which takes on its maximum value of 1 for the blackbody radiator. Because IR radiation is emitted by both sides of the leaf, a factor 2 is necessary to describe the energy loss by leaf as thermal radiation:
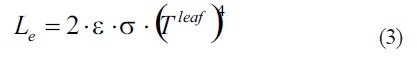
For plant leaves the emissivity is around 0,96; as an example, if leaf temperature (Tleaf) is 31 C (304 K), Le wouldbe approximately 930 W·m-2.
Convection heat flux density (JCH): Though physically different, heat conduction and heat convection are jointly treated in reference sources, so will be done here and will be called convection heat flux density. At this time it is necessary to introduce the concept of boundary layerdbl, what is rather difficult if the students do not have dealt with the difference between laminar or turbulent motion of fluid mass. Anyway, taking into account the general expression for heat conduction as a transport phenomenon, the boundary layer can be considered as the space interval through which the heat conduction takes place; if the students are familiar with the phenomenon of turbulence, the definition of boundary layer is presented as the distance away from the leaf through which the air can be considered unstirred, or what is the same, through which the turbulent mixing is absent.
Here, the heat conduction/convection flux density (JCH), will be compute as:
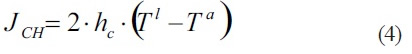
Where hC is the heat convection coefficient, Tl and Ta are the leaf and air temperature respectively.The factor 2 account for the fact than leaf exchanges heat through its two sides. For simplicity in problems and exercises Ta will be considered the same as Tsurr.
The value for hc is given by the ratio between the thermal conductivity of the air (Kair) and the boundary layer (δbl), while aerodynamical models are available for the latter, depending on the size and shape of the leaves and on the wind speed (Nobel, 2005). (For example of computing this term see problem below)
Transpiration heat flux density (JTH): Transpiration is the process by which water evaporates in some specific places within the leaves, and diffuses out. As evaporation is a cooling process, transpiration represents a mean of hear loss by a leaf. Conversely, a leaf can gain latent heat if dew or frost condenses onto it.
For this term to be introduced in the leaf balance equation, it is needed to consider first the flux density of water vapour, Jwv(mmol·m-2·s-1), then the transpiration heat flux density, JTH(W·m-2), that is going to enter the equation will be:

Where Hvap is the latent heat of water vaporization, 44,0 kJ·mol-1. The magnitude of Jwv can be given for make it easy the problem for the students. However, looking for a deeper insight of the learning objective, an expression for Jwv can be taken into account, as follow:
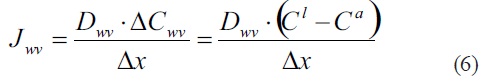
This expression is known as Ficks first law. Here Dwv is the diffusion coefficient for water vapor, cwv, mol.m-3 is the drop in water vapor concentration over some effective total distancex, expressed as the different between the water vapor concentration within the leaf (cl) and the corresponding of the air (ca).
This term of the leaf energy balance equation makes use of two important physical constant: the latent heat of water vaporization (Hvap), and the diffusion coefficient of water vapor in air (Dwv).Though both of these are temperature dependent, for the relevant temperature intervals for the applications of the equation, they can be treated as constant. Reasonable values for (Jwv) are between 2 and 5 mmol.m-2·s-1. Taking into consideration that the water vapor concentration within the leaf (cl) is near the saturated values, realistic figures for this variable range between 0,4 mol·m-3 (for 6 °C) to 2,2 mol·m-3 (for 35 °C). Remembering that relative humidity is a percent expression for the relation of ca to cl, it is not too difficult to put restrictions to the range of variation of ca. The effective total distance along with the transpiration occurs varies widely due to the differences in anatomical features of different leaves, so values of the order of magnitude of micrometers are recommended.
In this analysis the magnitude of amount of water, with the units of mol (or submultiples) are used. Units of mass, of course, are possible for that issue; in this case, the values of the constant Hvap changes accordingly.
It is important to make it clear that both heat fluxes (convective and accompanying transpiration) are most often positive, but they can be negative too. The heat flux for convection will be negative is the leaf temperature is lower than the air temperature, in such a case the leaf absorbs heat from the surroundings. As leaf losses energy due to the transpiration process, the opposite, i.e., the condensation of water vapor on the leaf makes the leaf to absorb energy from the environment; so occurs when dew appears on the leaves surfaces near dawn. (For example of computing this term see problem below)
The following problem illustrates the suitability of the LEBM for being used in problem solving practice:
Problem. A leaf, with solar radiationabsoptivity equals to 0,5 and inclination of 30° is exposed to a direct sunlight of 600 W·m-2 irradiance, so that its temperature reach 31° C. The effective temperatures of the sky and the surroundings are 15° C and 25° C respectively. Suppose that the leaf is in equilibrium with the environment and suggest how the leaf energy balance can be achieved, giving values for the emitted infrared radiation, beside convection and transpiration heat flux densities. Consider typical values for the flux density of water vapor between 2 and 5 mmol.m-2·s-1. Take for the emissivity and infrared radiation absorptivity 0,96.
Solution: In this problem the given data determine the amount of energy input to the leaf: as was shown above, for these conditions absorbed solar irradiation amount to 459,8 W·m-2; and absorbed infrared to 803,7 W·m-2, what gives a total energy input to the leaf of 1264,5 W·m-2. Being the leaf temperature given, the emitted radiation is determined, to 929,8 W·m-2. It is the time when the student realizes (might realize) that the terms convection heat flux density and transpiration heat flux density are not determined by given data. Then the student can give a value of 4 mmol·m-2·s-1 for the flux density of water vapor (Jwv), in such a case, the transpiration heat flux density (JTH) would be 176,0 W·m-2. This means that the convection heat flux density (JCH) must equals to 158,2 W·m-2. Since leaf and air (surroundings) temperatures are given, for this value of JCH to be achieved, the heat convection coefficient (hc) must equals to 26,3 W·m-2·°C-1. This is not an exclusive solution; other could be giving Jwp a smaller value, let us say2 mmol·m-2·s-1. In such a case, JTHwould amount to 88 W.m-2; for what 246,0 W·m-2 of is needed (energy balance is taken for granted), then hcmust be 41 W·m-2·°C-1, an extremely high value for this variable.
In this instructional experience, problems on the LEBM are set in the Physics course in the last unit, generally titled Quantum Physics, when the Stefan_Boltzmann law for the blackbody radiation is taught. The procedure for solving problems begins analyzing a real_life situation for a plant leaf on specified environmental conditions. Values for some magnitudes appearing in the LEBM equation are given, as usually in solving problems, and several others are asked for in a situation where leaf temperature is constant, i.e., stationary equilibrium condition. In such a state, the energy into the leaf is the same as the energy leaving the leaf.
It is easy to see that it is this feature of asking for several values as the solution is the score of the process that it is supposed to incite the creativity of the students (Domínguez et al., 2011). In the same way, the restrictions impose to the solution giving realistic intervals for the variables to be calculate is especially important for the students pursuing a formative profile related to agricultural sciences, since the agricultural engineer must face this type of professional problems: when a solution is needed in a mesh of restrictions.
The LEBM integrates contents of different Physics theories, i.e., gives the possibility of incorporate in one problem contents of different themes within one course or of different courses within the Discipline. In that purposethe heat convection term gives the possibility for revisiting the heat exchange as a transport phenomenon, usually studied within Molecular Physics theme, and the heattranspiration term introduce the possibility of a real application of change of state within the professional framework of the agricultural science.
In principle, computing the leaf T given all other variables is possible as an exercise, but its presence as a fourth power in the emitted radiation term makes the solution very difficult from because of the mathematics involved. However, a Microsoft Excel spreadsheet, free downloaded from internet was supplied to the students interested in that (Souza, 2003). This resource not only computes the leaf temperature, but allows the use of several details involved in the transpiration term not considered in the basic application.
REFERENCIAS BIBLIOGRÁFICAS
1. CILDIR, S. & N. SEZEN: Skill levels of prospective Physics teacher on Problem Posing, H.U. Journal of Education, No. 40:105-116, 2011.
2. DOMINGUEZ, M. J.; E. VELASCO; E. SANCHEZ; L.R. PARRA; J. MONTOYA: Activación de la cultura de la autoformación en carreras de perfil agrícola basada en problemas de física que estimulan la creatividad del estudiante. Revista Ciencias Técnicas Agropecuarias, 21(3): 79-83, 2012.
3. DOMINGUEZ, M. J.; E. VELASCO; E. SANCHEZ; J. MONTOYA: Procedimiento didáctico para desarrollar la capacidad creativa de los estudiantes de la carrera de Medicina Veterinaria, En: REDVET Rev. ElectronVet [en linea] Disponible en: http://www.veterinaria.org/revistas/redvetn121221/1211106.pdf [Consulta: agosto 15 2011].
4. GARCIA, J. & A. GARCIA: Teoría de la Educación.II: Procesos primarios de formación del pensamiento y la acción. (Manuales Universitarios). 385 pp., Salamanca, Ediciones Universitarias de Salamanca, España, 2001.
5. GARRET, R.M.: Problem solving in Science Education, Studies in Science Education, No. 13: 70-95, 1986.
6. NOBEL, P.S.: Physicochemical and Environmental Plant Physiolog, 3rd Ed. 567 pp., New York, Elsevier Academic Press, USA, 2005.
7. SEVRIN, T.: Open_ended problems in Physics. Upper secondary technical program students ways of approach outdoor physics problems. [en línea], Disponible en: http://www.umn.diva-portal.org/smash/get/diva2:505464/FULLTEXT01.pdf [consultado: Mayo 10, 2013].
8. SELCUK, S.; S. CALISKAN; M. EROL: The effect of problem solving instruction on Physics achievement, problem solving performance and strategies use. Lat. Am. J. Physics Educ., 2(3): 151-166, 2008.
9. SOUZA, A.:Tleaf2: Leaf energy balance simulation program. En: Laboratory exercise for plant ecophysiology [en línea]. Disponible en: http://www.ib.berkeley.edu/courses/ib151/Lab.htm [consultado: Septiembre 2010].
10. STINNER, A.: From Theory to practice: Placing contextual science into classroom, [en línea] University of Manitoba . Disponible en: http://www.umanitoba.ca/outreach/crystal/physics%20resources/From%20Intuitive%20Physics%20to%20Star%20Trek%202008-lcp.[consultado: Septiembre 2008].
Recibido: 12 de septiembre de 2012.
Aprobado: 5 de septiembre de 2013.
Juana Domínguez Mora. Universidad de Granma, Apartado Postal 21, Bayamo, Granma. CP: 85100. Cuba. Correo electrónico: jdominguezm@udg.co.cu














