INTRODUCTION
Sugarcane (Saccharum officinarum L.) is one of the main plants with a wide range of use in human food, cosmetics, pharmaceuticals, sugar, and alcohol manufacture. Besides, serving as an important source of energy in Brazil and the world. The sugarcane deserves to be highlighted in the national and international scene as a plant cultivated in the most diverse regions, and especially in the Center-South region of Brazil.
Food and Agricultural Organization of the United Nations (FAO) data for 2017 pointed to a world area harvested with sugarcane of 25 976 939 ha and a production of 1 841 528 386 t, and South America responded with a harvested area of 11 524 587.00 ha and production of 851 390 783 t. Of these, Brazil have a harvested area of 10 184 340 ha and a production of 758 548 292 t (FAO, 2019). In 2017, the Brazilian states that stood out was São Paulo (5 685 946 ha), Goias (922 817 ha), and Minas Gerais (906 464 ha), respectively (UNICA, 2019). Projections for the next ten years (2018-2027) indicate that 83% of the increase in sugar output is projected to originate in developing countries. Besides that, major changes in global production are expected in India +20%, followed by China +11%, Brazil +11%, Thailand +9%, and the European Union +5%. Brazil is projected to remain the main producer, although its sugar sector could face increased competition from the use of sugarcane for ethanol. Slower growth of production compared to the previous decade is foreseen in Asia (India, Pakistan, and Thailand) and Europe, which explains the slower annual growth in global sugar production over the outlook period +1.5% compared to the previous decade +2.0% (OECD-FAO, 2019).
One of the preponderant factors for the success of sugarcane cultivation and sugar production is the phytotechnical, sanitary and productive features of the stem and consequently of the seedlings. Especially, when working with asexual or vegetative propagation, the successive crops, which results in degeneration of the plant material used in the propagation and, consequently, a lower seedling. Besides, initial growth of reduced buds and tillers, delay of production, susceptible to the attack of pathogens organisms, with reduced production and final yield of sucrose (Jain et al., 2007; Viswanathan, 2016).
More advanced technologies for the production and management of sugarcane seedlings can result in rapid initial growth and development of shoots and tillers, rapid onset of production, less susceptibility to pests and diseases, high productivity, and higher commercial and industrial features of sugarcane. Such a claim could be confirmed by the use of plant growth regulators (PGRs). In particular, biostimulants, with pronounced physiological effect, which promote rapid growth and early development in the sugarcane seedling production phase (Karthikeyan and Shanmugam, 2017; Silva et al., 2017).
Plant growth regulators (PGRs) are substances analogous to plant hormones. Hormones are natural chemical messengers produced by a cell or tissue that modulate cellular processes in other cells by interacting with specific proteins, called receptors, that act on the cell transduction pathway. The plant hormones are synthesized at low concentrations and act at different plant locations, controlling plant growth and development (Taiz and Zeiger, 2010) being very relevant auxins, cytokinins, and gibberellins. In the case of PGRs, are represented by plant hormones (as well as, some plant extracts with hormonal action) or their synthetic analogs, by inhibitors of hormone biosynthesis or translocation and by hormone receptor blockers. Various plant developmental processes can be actively regulated in cultivated plants like acceleration or delay of seed germination, dormancy breaking in woody perennials, including fruit ripening and defoliation. In addition, the important sugarcane processes that could be mediated and regulated by PGRs. Among them, mainly, stimulation or reduction of shoot elongation, induction or reduction of flowering and fruiting, reduction or increase of fruit set and acceleration or delay of senescence processes. The achieved benefits range from facilitating crop management to increasing, securing yield of the harvested produce, improving its storage and shelf life, beyond product quality (Rademacher, 2015; Terefe et al., 2017; Nguyen et al., 2019).
These substances are used in sugarcane in vitro and in field culture. For instance, auxins and cytokinins play an important role in the induction of calluses, budding and root regeneration (Gopitha et al., 2010), and rapid in vitro culture and micropropagation (Roy and Kabir, 2007; Behera and Sahoo, 2009). Associated with these PGRs, gibberellins are also preponderant factors in callus formation and regeneration of sugarcane plant material (Dash et al., 2011).
Instead, previous results showed that PGRs are widely used in modern agriculture, be like in horticulture and viticulture. In this sense, Stimulate® is a commercial biostimulant that contain a mixture of plant growth regulators in the form of the liquid solution that has been used in sugarcane cultivation in Brazil. Authors as Miguel et al. (2009) showed that the profitability index with the use of Stimulate® in the seedlings (26.22%) and foliar (25.48%) were superior that the control (13.09%). Moreover, the application of 0.5 l ha-1 Stimulate® (carried out at planting) in the seedlings combined with phytosanitary treatments at planting resulted in higher productivity and, consequently, a higher profitability index. In addition, the positive effect of the product can also be confirmed by Silva et al. (2010) testing the Stimulate® at a dose of 0.5 l ha-1 and liquid fertilizers during the cultivation of different sugarcane cultivars at 70 days after the fourth harvest, showing favorable effect in increasing yield of stalks, sugar, and longevity, independently of the cultivars of sugarcane.
Physiologically, the propagation and cultivation of sugarcane presents a slow phase of budding and, subsequently, a tillering phase and very slow growth to start an intense tillering phase (maximum) and, consequently, grow and develop faster (Santos and Borém, 2016). The use of the plant growth regulator (biostimulant) can be an option to adjust and provide greater development in this slow initial phase of seedling production that will start a new commercial planting and start a new productive cycle. Another predominant factor in cultivation is the difference in growth provided by cultivars with the potential for greater development in the initial seedling production phase and, possibly, later planting in the field. Therefore, it is essential to evaluate the different cultivars in the initial seedling production phase, identify promising cultivars, and subsequently, study them in the field, avoiding unnecessary expenses and prioritizing the time of seedling and cultivation, maximizing yields.
The early evaluation of the PGRs effects on commercial cultivars growth could be a useful tool for technical assisting and orientation to farmers. Taken into account these considerations, this work aims to determine the effect of plant growth regulators (present on Stimulate® biostimulant) on the growth of seedlings of two commercial sugarcane cultivars in greenhouse.
MATERIAL AND METHODS
Plant material
Seedlings of CTC 04 and CTC 9002 were used. It came from healthy plants, without pests and diseases. These two commercial cultivars are used in the sugar and alcohol mills of the Triângulo Mineiro region, in Brazilian Center-South. Both cultivars were developed by The Sugarcane Technology Center (Centro de Tecnologia Canavieira, CTC). CTC 04 presents important characteristics such as the medium maturation cycle, excellent tillering and closing, is born very well in the mechanized planting and it has a very good sprout growth under the straw, besides to tolerate drought well, with stability and longevity. Already the CTC 9002 shows a medium maturation cycle, drought tolerant rusticity in the Cerrado, with erect growth habit.
Besides, it were used mini toilet (follow-up stem) of sugarcane cultivars CTC 9002 and CTC 04 removed from growing crops. As used by farmers, containing two buds, 20 cm of length and approximately three centimeters of diameter.
Plant growth regulators (PGRs)
The plant regulators used was those included in the Stimulate® biostimulant (commercial product) belonging to Stoller do Brasil LLC. that containing: 0.009% kinetin (cytokinin), 0.005% gibberellic acid (GA3), 0.005% indole butyric acid (auxin) and 99.981% inert ingredients.
Experimental conditions
The experiment was conducted in a greenhouse at Universidade do Estado de Minas Gerais-UEMG, Campus of the Ituiutaba, Minas Gerais State, Brazil. The geographical coordinates of the site are latitude 180° 58' 19.1" South; longitude 490° 26' 50.5" W GrW and altitude of 600 meters. The natural vegetation is represented by the Cerrado and the climate, according to the classification of Köppen is the Aw type, tropical savannah climate, with dry winter and rainy summer. In general, the temperature of the coldest month is above 18 °C and the driest month of the rainfall is less than 60 mm. During the experiment, the climate was characterized by an absence of precipitation (mm) and, generally, by high temperatures and low relative humidity as shown in figure 1.
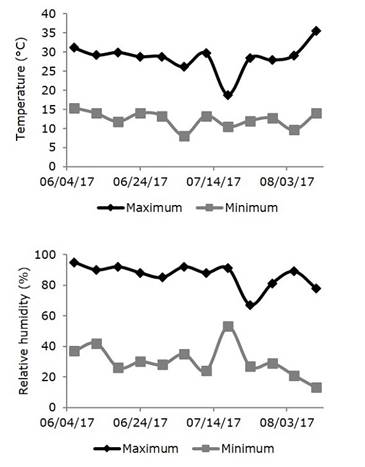
Figure 1. Temperatures and relative humidity (maximum and minimum) during the experiment from 06/04/17 to 08/03/17. Data were provided by INMET (2017) .
The experiment was carried out in a randomized block design in factorial scheme (2 x 6) with 12 treatments, two commercial sugarcane (Saccharum officinarum L.) cultivars (CTC 04 and CTC 9002) and six concentrations of plant growth regulators (commercial product). The experiment was distributed in four blocks (four repetitions), with four seedlings, and with a mini toilet (follow-up stem) of sugarcane removed from growing crops, as is usually done by farmers.
The seedlings was treated with 8 ml l-1 sodium hypochlorite solution (2.0 to 2.5% v/v) active chlorine applied for 10 minutes and rinsed with distilled and deionized water in abundance as recommended for domestic used as a vegetable sanitizer (Five-hundred milliliters of a solution).
The selection and preparation of seedlings was made for planting in polyethylene plastic bags (34 cm long, and 27 cm wide). After initial preparation, the stalk segments (seedlings) was inserted into substrate (in a ratio of 1:1:1 v:v:v, prepared with 1/3 gully soil, 1/3 of coarse sand washed, 1/3 of tanned bovine manure) + 5 g l-1 of the fertilizer formulation 04-14-08 + 1 g l-1 of dolomitic limestone, according to the recommendation for soils of Cerrado (Oliveira, 2016) for the composition of the rooting substrate to seedlings.
The bags remained in a greenhouse with an area of approximate 24 m2, polyethylene cover (120 μm), 50% shading in side and 2.0 m height. All the plant material was irrigated daily for the whole period of the experiment, as measured in the laboratory until the field capacity.
Approximately 30 days after planting it was performed the selection and standardization of seedlings by size and number of leaves. Two completely open and developed leaves were leaving and the rest were removed. Later, the regulators containing in a biostimulant was applied directly to the leaves with hand spray aid, cone tip and plastic curtain between treatments to prevent drift.
The treatments studied was: control (no application), biostimulant 0.5 ml l-1; 1 ml l-1; 2 ml l-1; 4 ml l-1; 8 ml l-1 of commercial product per liter of solution in distilled and deionized water. According to previous test to define and standardize the amount of solution per treatment, five-hundred milliliters of solution were used. Spraying was performed after 5:00 p.m. to avoid loss of product and or drift.
After treatments application, the following evaluations was carried out at weekly intervals: plant height, leaf number, number of internodes and number of shoots. The plant height (PH) was a biometric measurement performed using a ruler graduated in centimeters. It was considering the distance from the ground level (base of the plant on substrate) to the tip of the apical bud (highest point of the plant stem) as the height of the plant. The number of leaves per plot (NL) was performed by manually counting the total number of leaves in the shoots, starting at the base of the stem to its apex. The number of internodes (NI) was determined by counting on the internodes in the stems, starting at the base of the stem to its apex.
In addition, 28 days after application, the following variables was evaluated: plant height, number of internodes, stem diameter, fresh and dry mass of shoot, and root. The height of branches was a biometric measurement carried out with the help of a scale graded in centimeters, considering the height of the branches the distance from the soil level until the atrial region of leaf +1. The number of internodes (NI) was determined by counting the number of internodes of the stems, starting at the base of the stem to its apex. Stem diameter (SD) was determined in the 3° internode above the surface of the ground, with the aid of a pachymeter graduated in centimeters. The fresh mass of the aerial part (FMAP) was performed with the manual separation of all aerial part produced in the seedlings (shoots and/or tillers) and weighing by means of a precision scale, in mg. The fresh mass of the root system was determined with manual separation of the entire root system produced in the seedlings and weighing by means of a precision scale, in mg. Subsequently, the FMAP and FMRS was dried in a drying oven with the forced air chamber at 60 °C until the constant mass of the plant material, obtaining the dry mass of aerial part (DMAP) and dry mass root system (DMRS).
Statistical analysis
The data was submitted to the normality test (Shapiro-Wilk) and homogeneity test (Cochran), analysis of variance by the F test, and the averages compared by the Tukey test at 5% probability (p=0.05). In addition, a regression study of the tested concentrations and selecting variables was performed. The statistical analysis program used for data processing was Sisvar (5.6 version).
RESULTS AND DISCUSSION
The data of the evaluated variables had normal distribution according to the normality and homogeneity tests of the variances performed. The results showed that at concentrations of the commercial biostimulant assayed, neither at 7 or 28 days after foliar application, the media valor of the variables in each cultivar were not different to the control (Tukey test p=0.05) (Supplementary material). Nevertheless, the regression analysis of all data of the selecting variables (plant height, stem diameter, number of internodes, root system fresh and dry mass) demonstrated the effect of concentrations. The regression equations and the determination coefficient revealed the representativeness of the data (all above 80%) graphically and explained the relation between the plant response and the concentrations (Figure 2).
Plant height (R2=0.9822) and the number of internodes (R2=0.8127) decreased proportionally to the increase in plant growth regulators concentrations present in the Stimulate® biostimulant (Figure 2 A and C). In the same line, a quadratic response to increase concentrations for root system fresh mass (R2=0.9869) and dry matter (R2=0.8897) (Figure 2 D, E) were observed.
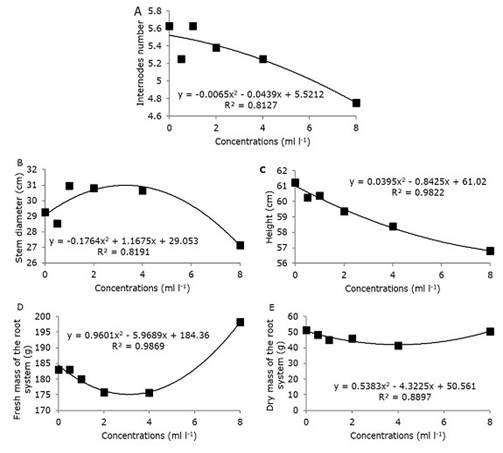
Figure 2. Growth indicators of sugarcane plants treated with different concentrations of plant regulators found in the Stimulate® biostimulant, 28 days after application. Number of internodes (A), stem diameter (B), plant height (C), fresh mass of the root system (D), dry mass of the root system (E).
On the other hand, the diameter of the sugarcane stem (R2= 0.8191) (Figure 2 B) increased proportionally to the increase of the concentrations up to 4 ml l-1 and, subsequently, decreased with the highest concentration (8 ml l-1), demonstrating the possible phytotoxic effect of the latter.
While some improvement would be possible by more concentrations and cultivars tested in greenhouse studies, the results demonstrated that at this level is possible to early detect some effect of the biostimulant Stimulate® on sugarcane plant growth. Although some studies indicated positive effect for it application in the field (Miguel et al., 2009; Silva et al., 2010), in not all case the increments of plant growth and developments achieve the expectative. In this sense, Oliveira et al. (2013) using a mixture of several commercial products with the biostimulant revealed that the application of Stimulate® + Regent® + Comet® + Starter® did not have an effect on the productivity and the technical variables of sugarcane.
In this scenario, the finding in greenhouse test that variables as number of internodes (Figure 2 A) and plant height (Figure 2 C), accompanied by stem diameter (Figure 2 B) decreased with high biostimulant concentration tested, demonstrating the inhibitory effect, or rather, growth retardation of the vegetative part. However, biostimulant promoted the root growth with increment on fresh and dry root mass (Figure 2 D and E) with increasing biostimulant concentrations. That can be positive for sugarcane cultivation, especially in regions (such as the south-central region of Brazil, characterized mainly by Cerrado biome), which suffer from weather conditions such as drought and lack of intense water. Reinforcing such arguments, Matsuoka and Garcia (2011) reported the importance of a well-developed root system for sustainable sugarcane production.
Additionally, is necessary to take in consideration that for the desired effect with the use of specific biostimulants, it is important to know the process regulated by the hormone or group of hormones (if synthetic or plant growth regulators), the concentration to manipulate the process, as well as the organ (or part) of the plant where the biological reactions will occur. Because of its composition of multiple hormones at low concentrations, as well as the small doses recommended, the isolated application of a biostimulant can hardly regulate or completely manipulate a physiological process. The biostimulant will be a complement to the aid of physiological maintenance, which can be very important in environmental conditions (drought, frost) or limiting biotic (pests and diseases) (Costa et al., 2011). Therefore, all these studies in the field are highly time and labor consuming, increasing the cost and it have the risk of affectation for environmental factor. According to that the results in greenhouse are useful tools. It is recommended that further studies be carried out with sugarcane cultivation, genetic variability and bioestimulants to ensure positive recommendation results in field.
CONCLUSIONS
The commercial biostimulant Stimulate® have negative effect on number of internodes, plant height and stem diameter and promote the root growth of two commercial sugarcane cultivars seedlings in greenhouse. The results point up the bases for doses adjustment, cost reduction and technical assistant to the farmers. It is recommended that further studies to ensure positive recommendation results in field.















