INTRODUCTION
Oroxylum indicum (L.) Vent. (Bignoniaceae) commonly known as Shivnak, Shyonak, Sonpatha or midnight horror, is a small deciduous, soft wooded tree. It is distributed throughout the India up to an altitude of 1200 m and it is found mainly in ravine and moist places in the forests (Bennet et al., 1992). This plant is a rich source of a broad range of secondary metabolites such as alkaloids, flavonoids, tannins, terpenoids, carotenoids and anthocyanin. Among them, flavonoids are the major storage components of O. indicum (Grampurohit et al., 1994; Chen et al., 2003). The plant is used in many ayurvedic preparations widely utilized by people for health care. The root bark of this plant is reported to be administered as astringent, bittertonic, stomachic and anodyne. Besides, it is an important ingredient in famous tonic formulations such as Chyawanprash (Ghate, 1999; Parle and Bansal, 2006), Dashmularishta, Narayan Taila, Bhrama Rasayana, Dhanwantara, etc. (Joshi et al., 2014).
On the other hand, dichloromethane extracts of the stem bark and root possess antimicrobial, antifungal, antinflammatory and anticancerous properties (Lambertini et al., 2004; Siriwatanametanona et al., 2010). This tree have flavonoids of great medicinal value. Among them, baicalein, one of the important flavonoid used to inhibit proliferation of human breast cancer cell line MDA - MB - 435 (Mao, 2002; Naveen et al., 2012), its derivative baicalein 7-0-glucoside and Ellagic acid in root bark, oroxylin A, chrysin and scutellarein in stem bark, are the most important potential active compound present in this plant (Harminder and Chaudhary, 2011). Previous work has already been done on flavonoid assessment from natural plant parts (Vasanth et al., 1991; Chen et al., 2003; Maitreyi et al., 2008; Zaveri et al., 2008; Samatha et al., 2012; Karnati et al., 2013, Samatha et al., 2013).
In order to preserve this endangered species and to use biomass as source of secondary metabolites, different authors developed efforts for in vitro culture of O. indicum. In this sense, direct in vitro regeneration (Gokhale and Bansal, 2009), indirect organogenesis (Gokhale and Bansal, 2010), micropropagation (Dwivedi and Boro, 2012) and callus induction (Rami and Patel, 2014; Samatha and Nanna, 2016) has been referred. As noted in other crops, callus culture is dependent of explant type and plant growth regulators (Rami and Patel, 2014; Samatha and Nanna, 2016).
Based on the knowledge gained from studying the phytochemical composition of in vitro grown roots (Gokhale and Bansal, 2009), callus and cell suspension (Gokhale et al., 2016), the presence of flavonoid, anthraquinone and phenol on in vitro cultures is a demonstrated scientific fact. Specifically, it was confirmed by the detection and extraction of the flavonoids baicalein and chrysin in fractions obtained using different solvents (Gokhale et al., 2016). Although considerable progress has already been made in this direction, the biological activities of callus and cells suspension extracts of O. indicum needs study and research. According to that, the aim of this work was to determine the in vitro antioxidant and antibacterial activities of O. indicum callus extracts.
MATERIAL AND METHODS
Plant material
Seeds of the Oroxylum indicum (L.) Vent were collected from matured trees of the reserve forest areas in and around Jabalpur, Madhya Pradesh (India) and surface disinfected.
Callus formation
To induce the formation of suitable callus, the selective medium (Gokhale et al., 2016) was used. The MS medium (Murashige and Skoog, 1962) was supplemented with benzyl amino purine (BAP) 1 mg l-1 and AgNO3 2 mg l-1. Different explants i.e. leaf, axillary bud, apical bud, root, embryonic axis of mature and immature seeds (size 0.5 cm) were excised from 15 days old seedlings of the O. indicum developed on MS medium. Explants were surface disinfected with ethanol (70% v/v) for 30 seconds and then immersed with 0.1% mercuric chloride solution for 1-2 minutes. Then, they were rinsed three times with sterilized distilled water. The disinfected explants were inoculated on solidified MS medium in culture tubes, each culture tube with one explant, under aseptic conditions in laminar hood.
The cultures were maintained in culture room at 25 ± 2 °C, relative humidity of 60-70% and a light intensity of approx. 1500 lux provided by cool, white, fluorescent tubes under a photoperiod of 16/8 h (light/dark).
Calluses from different explants of Oroxylum indicum were distinguished based on their characteristics such as texture, coloration, appearance and their fresh weight and dry weight. For growth measurement, the callus was harvested and the fresh weight (FW) (g) was recorded. After this, the callus was dried in an oven at 40 °C for 24 h and the dry weight (DW) (g) was determined.
Metabolites extraction and characterization
Extraction
The extraction of metabolites was carried out from two-month-old callus developed on MS medium. The ethanolic and aqueous extracts were prepared in cold and hot conditions following the methods proposed by Stalikas (2007) with few modifications, 5.0 g of the oven dried powdered callus were mixed in 100 ml of 70% ethanol in 250 ml conical flasks. Extract was incubated in an orbital shaker for 24 hours on 110 rpm. After incubation period, extract was filtered with Whatman filter paper under aseptic conditions (cold extract). Besides, the extract was concentrated on hot plate for 8 min at 120 °C (hot extract).
In the case of aqueous extract, dried powder of callus was mixed in sterilized distilled water and kept on orbital shaker at 70 rpm for 48 h at room temperature (cold callus extract) or by mixing dried powder of callus in sterilized distilled water and concentrated on hot plate for 6 min at 120 °C (hot callus extract).
The metabolites that were present in callus extract of O. indicum were detected and quantified by standard procedures.
Phenolic and flavonoid determination
The aqueous extracts (cold and hot) were used to phenolic and flavonoid content determination.
The amount of total phenolic compounds in extract of callus culture was estimated with the Folin-Ciocalteau reagent using the method of Spanos and Wrolstad (1990) modified by Lister and Wilson (2001). The content of total phenolic compounds expressed as µg g-1 Gallic Acid Equivalent (GAE) of dry extract according to standard curve (y = 9.53x - 0.13, R2 = 0.996) of gallic acid (0-250 µg ml-1).
The total flavonoid content of callus extract was determined by the aluminum chloride colorimetric method (Chang et al., 2002). The total flavonoid content was calculated as mg g-1 from a calibration curve and the result was expressed as mg Quercetin equivalent (QE) per g dry weight.
Spectroscopic analysis (UV-Vis and FTIR)
Extracts of callus were subjected to thin layer chromatography (TLC) as described by Gokhale et al. (2016) using solvent systems Methanol: Water (70:30). Besides, UV -Vis spectrophotometry (range 200-600nm) was used to check the presence of flavonoid (baicalein). For comparative analysis of baicalein presence, standard was prepared as 0.5 mg of Baicalein (Sigma-Aldrich) in 1 ml ethanol.
Fine powder of oven dried (40 °C) callus was mixed with Potassium bromide (FTIR grade) in the ratio of 1mg:10 mg as described by Visveshwari et al. (2017).The powdered sample was loaded in Fourier Transform Infrared Spectrometer (FTIR) (Shimadzu, IR affinity 1S) to detect the characteristic peaks and functional groups in the range of 400-4000 cm-1 .
In vitro activities of callus extract
Antioxidant activity
The free radical scavenging activity of the callus ethanolic and aqueous extracts were investigated using 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging method as reported by Karadag et al. (2009). The assay mixture contained 2 ml of 1.0 mM l-1 DPPH radical solution prepared in methanol and 1 ml of standard or extract solution of different concentrations (1-7 µg ml-1). All data were compared with the values of standard ascorbic acid curve and expressed as µg ml-1 equivalent.
Antimicrobial activity
Well diffusion method: The ethanolic extract (hot) was used for testing their antibacterial activity against two species of pathogenic bacteria Micrococcus luteus (MTCC 7950) and Staphylococcus aureus (MTCC 9542), collected from Microbial Type Culture Collection and Gene Bank, Chandigarh, India. The antimicrobial activity was performed by agar well diffusion method (Stepanovic et al., 2003). Nutrient agar plates were prepared and swabbed with pure culture of bacterial strains. The plates were kept for incubation for 20 minutes. After the incubation period, four wells (7 mm diameter) were punched in the plates using a sterile stainless steel borer. One well was filled with 50-60 µl of callus extract, second well was filled with distilled water as negative control, third well was positive control with disc of antibiotic (Streptomycin, 10 mcg/disc) and fourth well was filled with ethanol. The plates were then kept for incubation at 37 °C for 24 hours. Each experiment was set in three replicas and the diameter of inhibition zone (mm) was compared against the inhibition zone around antibiotic disc.
MTT Assay: MTT assay viability tests were carried out using a commercially available cell proliferation reagent 3-(4,5-dimethylthiazolyl-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) (135038, Sigma, France). The ethanolic extract (hot) was prepared as describe above with callus of 4, 5 and 6 month of culture.
Experiment was performed with bacterial culture of Staphylococcus aureus (MTCC 9542). It was culture in nutrient broth medium and incubated at 37 °C for 24 hours. After, 180 µl of bacterial culture was mixed with 40 µl of ethanolic callus extract (hot) in flat-bottom 96-well plates (831835, Sarstedt, France). Bacterial culture without callus extract was used as control.
After overnight incubation (37 °C), stock MTT solution (5 mg ml-1 MTT in PBS) was added (20 µl per well) and plates were incubated in a humidified atmosphere with 5% CO2 at 37 °C for half an hour. Then, insoluble purple formazan was dissolved by adding 100 µl dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO, USA) to each well, and incubated for 4 h more. The absorbance at 540 nm was measured with a reference wavelength of 690 nm, using an ELISA reader (Alere AM 2100 Microplate Reader) (Fontanay et al., 2008). Cell viability rate was calculated as the percent (%) of MTT absorption as follows: Cell survival = (Mean experimental absorbance/Mean Control absorbance) x100.
RESULTS AND DISCUSSION
Callus Formation
Among various type of explants, callus were formed from three explants viz; root, embryonic axis of mature seed and embryonic axis of immature seed. After 14 days of culture, callus initiated from root, embryonic axis of immature seeds was soft and delicate, and callus developed from embryonic axis of mature seed was bulky, friable and dark brown in color (Figure 1 a, b, c). In addition, the shoots regeneration was observed over the callus surface (Figure 1 d).
The combination of 1 mg l-1 BAP and 2 mg l-1 AgNO3 on MS, was suitable for callus formation after 14 days in this selective medium (Gokhale et al., 2016). The rate (mass/time) of callogenesis was optimum and callus had in average fresh weight of 1.063 ± 0.04 g and dry weight of 0.795 ± 0.0 g.
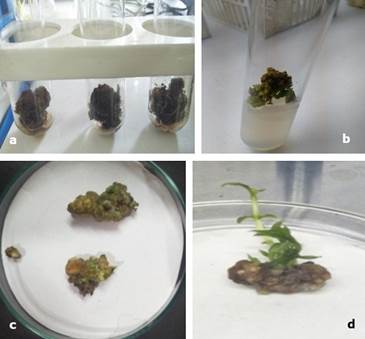
Figure 1. Callus of Oroxylum indicum on selective medium (MS, 1 mg l-1 BAP and 2 mg l-1 AgNO3). a- Dark brown and friable callus grown, b- green callus turning to brown bulky, c- callus without shoot, d- Callus with shoots regeneration.
The combination of BAP and AgNO3 resulted in the induction of embryogenic/rhizogenic calluses in O. indicum. This results corroborate previous finding (Gokhale and Bansal, 2009; Gokhale and Bansal, 2010; Gokhale et al., 2016). There are several examples of induction of organogenic calluses and direct regeneration in response to BAP (Ali et al., 2008). Nevertheless, in other studies it is noted that different plant growth regulators has been used for callus induction in O. indicum. For instants, Rami and Patel (2014) indicated that 2,4-Dichlorophenoxyacetic acid with BAP was more effective for callus induction, whereas, Samatha and Nanna (2016) found that among the various auxins used, Indole butyric acid (IBA) and 2,4-D were more potent for callus proliferation followed by Indole acetic acid/Naphthalene acetic acid.
Metabolites extraction and characterization
The presence of phenolic compounds was demonstrated in aqueous callus extracts. The cold extract yielded 10.27 ± 0.5 µg ml-1 GAE (Gallic acid equivalent/g), whereas the hot extract yielded 9.1±0.8 µg ml-1 GAE.
Comparing with related scientific literature, in which determination of phenolic content were reported, Yen and Chuang (2000) found 180.64 ± 6.51 mg GAE g-1 in water extracts of Cassia tora L. Adebooye et al. (2008) informed 0.704 mg GAE g-1 fresh weight of Solanum nigrum L. in water extract and Lee et al. (2015) reported a total phenol content of 56.8 ± 5.9 mg GAE g-1 fresh weight of Alternanthper asessilis and 36.4 ± 6.1 mg GAE g-1 fresh weight of Ipomoea aquatica in acetone-water-acetic acid extracts. Nevertheless, the presence of phenolic compounds in O. indicum callus obtaining on in vitro conditions demonstrated the potential of tissue culture techniques for producing secondary metabolites of this important medicinal species.
Flavonoids are secondary metabolites with antioxidant activity, the potency of which depends on the number and position of free OH groups (Panche et al., 2016).In present studies the flavonoid content of cold and hot callus extracts was 139.16 ± 7.9 µg ml-1 QE and 181.6 ± 6.3 µg ml-1 QE, respectively. The genetic diversity, biological, environmental, seasonal and year-to-year variations significantly affects the flavonoid content of vegetables (Kumar and Roy, 2018). In despite of, those problems not take place with in vitro culture.
The results of the present study has coincidences with others reports about the presence of flavonoid and phenol contents in ethanolic extract of stem bark as well as in callus and cell suspensions of O. indicum (Kalaivani and Mathew, 2009; Gokhale et al., 2016).
Spectroscopic analysis
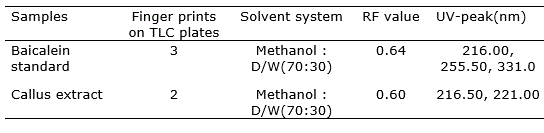
TLC results revealed the presence of flavonoid as baicalein in the callus of O. indicum. Rf value obtained of the standard was 0.64 and the callus extracts having Rf values of 0.60, when a solvent phase of methanol: distilled water (70:30 ml) was used.
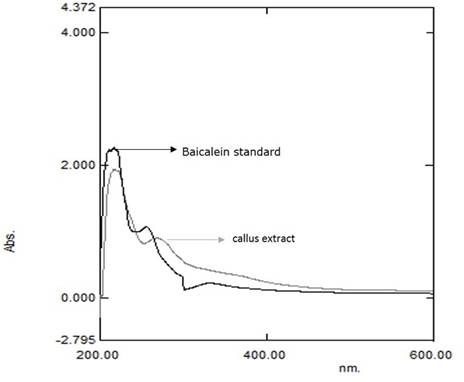
The qualitative UV-Vis spectrum profile of ethanolic extracts of O. indicum was selected from 200-600 nm due to sharpness of peaks and proper baseline. The profile showed the peaks from 200 to 600 nm. Standard (baicalein) offered three spectral peaks (216.0, 255.0 and 331.0 nm). All the extracts provided many spectral peaks, which were obtained at different absorbance. However, one peak, which is mentioned here, was at 216.00 nm in all the extracts that coincide with the peak of standard (Table 1). According to that, the result of UV-Vis spectroscopic analysis confirmed the presence of baicalein in ethanolic extracts of O. indicum (Figure 2).
Spectroscopic methods have become a powerful tool for secondary metabolite profiling as well as for qualitative and quantitative analysis of the pharmaceutical and biological materials (Janakiraman and Jeyaprakash, 2015). The present study of UV-Vis spectrophotometer revealed the presence of the flavonoid compound that indicates the potential of in vitro culture to produce medicinal compounds from this plant. Isolated flavonoid, baicalein acts as antioxidants, hepatoprotective, anti-inflammatory and as anticancer (Mao, 2002; Naveen et al., 2012).
FTIR analysis
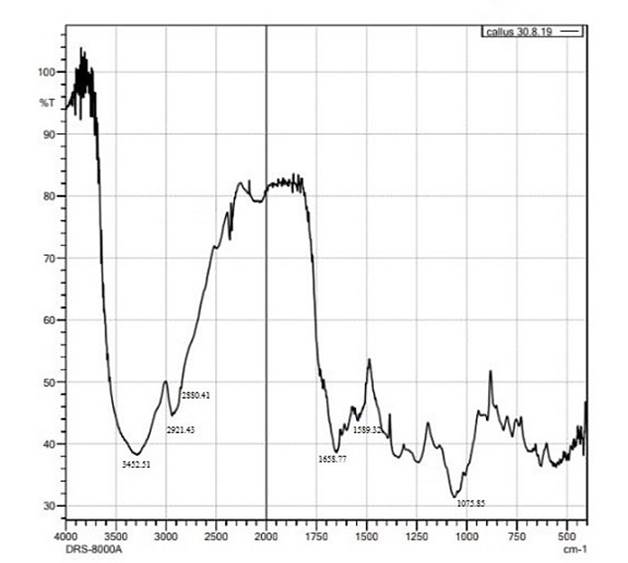
FTIR analysis of callus revealed the functional groups present in the spectrum of the baicalein (Xie et al., 2017) based on the peak values in the region of infrared radiation. FTIR studies enable the identification of the chemical constituents and elucidation of the structures of compounds. The major bands were observed at 3452.51, 2921.43, 1658.77, 1589.32 and 1075.85 cm-1 (Figure 3). The peak at 3452.51 cm-1 indicates the O-H stretch that might be due to the presence of phenols and alcohols and 2921.43 cm-1 corresponds to the strong C-H stretch. The bands at 1658.77 cm-1 confirms the presence of aromatic carbonyl group, 1589.32 cm-1 indicates C=C and 1075.85 cm-1 corresponds to C-O. In addition, some weak absorption bands were also recorded in the spectra. FTIR is an important tool for plant extract analysis. The presence of functional groups revealed by analysis provides the scientific basis for the therapeutic properties of the plant and thus recommend to use it on the basis of phyto-pharmaceutical importance (Omotoso et al., 2014). In relation with this point of view, Rajiv et al. (2017) by FTIR analysis concluded that the methanolic extract of Myristca dactyloides has potential bioactive compounds like alkaloids, glycosides, flavonoids, and tannins. All the extracts of M. dactyloides with these functional groups have medicinal properties and can be used as antimicrobial and anticancer agents.
In vitro activities of callus extracts
Antioxidant activity
The radical scavenging activity of the extract was observed by the decrease in absorbance of the DPPH at 514 nm. This is manifested in the rapid discoloration of the purple DPPH to light yellow, suggesting that the radical scavenging activity of extract was due to its proton donating ability (Boussaada et al., 2008). This confirmed the presence of flavonoid compounds in extract with the reduction of DPPH. Ethanolic callus extracts of O. indicum were more effective free radical scavenger or hydrogen donor that contributes significantly to the antioxidant activity (Figure 4). The extraction procedures and solvents are responsible for dissolving the endogenous compounds of the plants (Siddhuraju and Becker, 2003). Phenolic compounds are important plant constituents with redox properties responsible for antioxidant activity (Soobrattee et al., 2005). Besides, the hydroxyl groups in plant extracts are responsible for facilitating free radical scavenging.
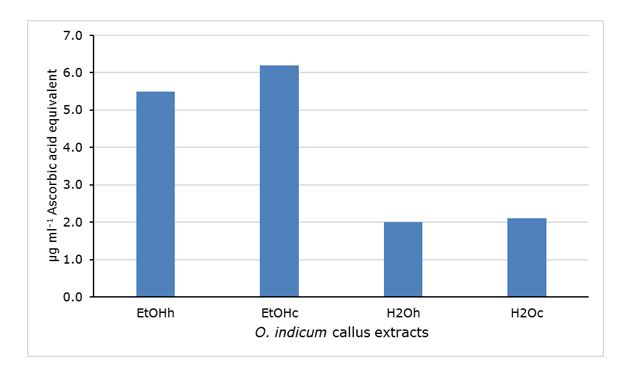
Figure 4. In vitro antioxidant activity of Oroxylum indicum callus extracts expressed as µg ml-1 Ascorbic acid equivalent. EtOH ethanolic extracts (h, hot; c, cold), H2O aqueous extracts (h, hot; c, cold).
Antimicrobial activity
It was demonstrated that the callus ethanolic extract (hot) had antibacterial effect against the strains assayed. The diameter of inhibition zone against Staphylococcus aureus was found to be the highest i.e, 17.5 mm (diameter). The clear zone formation in surroundings of well of Streptomycin disc was 6 mm (diameter) (Figure 5). In previous works, several authors demonstrated the significant antibacterial activity of methanol, acetone and water extracts of Centella asiatica Linn. callus against S. aureus and other tested microorganism (Sekar et al., 2011). Besides, agar well diffusion method had been successfully used in signifying the antibacterial activity of extracts of Aloe barbadensis Miller. (Aloe vera), Azadirachta indica (Neem), Bryophyllum etc. against S. aureus (Dahiya and Purkayastha, 2012).
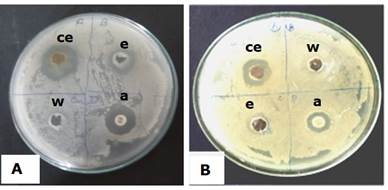
Figure 5. In vitro antibacterial activity of Oroxylum indicum callus extracts against Staphylococcus aureus (MTCC 9542) (A) and Micrococcus luteus (MTCC 7950) (B). a-Streptomycin disc, ce- ethanolic callus extract, w- water, e- ethanol.
The results of MTT assay corroborated the in vitro antibacterial effect of O. indicum callus ethanolic extract with reduction of the S. aureus bacterial cells viability. When it were treated with ethanolic extract of callus with different time of in vitro culture, the percentage of viability decrease lineally (R² = 0.9835) (Figure 6). Similar results on cytotoxic effect of four medicinal plant species were reported using MTT assay against Staphylococcus aureus and Enterococcus faecalis (Kudumela et al., 2018).

Figure 6. In vitro antibacterial activity of Oroxylum indicum callus ethanolic extract against Staphylococcus aureus (MTCC 9542) in MTT assay. EC- Extract of callus with 4 month (EC4), 5 month (EC5) and 6 month (EC6) of culture.
The isolated baicalein from roots of Oroxylum indicum is used to cure various human diseases (Doshi et al., 2012). As per increasing demand of root of O. indicum to isolate its metabolites, the plant has been uprooted and destroyed on large scale. In this research work, the presence of baicalein in callus of O.indicum as well as the antioxidant and antibacterial effects of callus extracts were demonstrated. So, the industrial production of this medicinally active compound would be implemented for the welfare of the society. As, the cost will be reduced on mass production of baicalein by in vitro techniques, it will be available to large number of people for curing various diseases. Additional investigations are needed to elucidate other biological activities of in vitro produced metabolites of O. indicum.