INTRODUCTION
Sugarcane, Saccharum officinarum L., is a leading cash crop cultivated in many parts of the world for commercial purposes, especially in sub-tropical and tropical regions of the world (Mirajkar et al., 2019). Worldwide, sugarcane is cultivated over a zone of 20.10 million hectares with a production of 1 318.10 million tones and productivity of 65.5 tons per hectare. Sugarcane area and profitability contrast broadly from country to country. Brazil commands the highest area (5.34 million hectares) while Australia has the highest productivity (85.1 t ha-1).
India positions second among the sugarcane developing nations of the world in both area and productivity after Brazil with an area of 4.94 million hectares under cultivation and average yield of 78 tons per hectare (Verma and Solanki, 2020). However, diseases caused by various fungi, bacteria, phytoplasma and viruses pose a serious threat to sugarcane cultivation in all the sugarcane growing regions of India (Rao et al., 2002; Viswanathan and Rao, 2011). Among the various fungal diseases; wilt, smut and red rot are of major economic importance imposing severe direct and indirect limitations to sugarcane cultivation throughout the country (Viswanathan and Rao, 2011).
Wilt of sugarcane was first reported in 1913 (Butler and Khan, 1913). Over the years various researchers have reported different fungal species to be associated with sugarcane wilt like Fusarium sacchari, F. proliferatum and F. moniliforme. However, Viswanathan et al. (2011) identified F. sacchari as the fungal species most frequently associated with wilt based on molecular studies. The predominant mode of transmission of this disease is through infected setts at time of planting, but the inoculum can also survive in soil for up to 3 years. Sugarcane wilt causes reduction in germination and in severe cases, total loss of cane yield may also occur due to drying up and wilting of the stalks. Agnihotri and Rao (2002) observed wilt as one of the major fungal diseases of sugarcane next to red rot in causing economic losses. Studies have reported that economic losses due to wilt can go up to 65% (Sharma, 1976). Besides reduction in cane yield, this disease also results in reduced juice extraction (15-30%) and sugar recovery (20%). Moreover, it has been observed that combined infection of red rot and wilt can cause much higher damage to crop than the individual infection of these diseases (Viswanathan et al., 2012; Bhat et al., 2016).
To date, the management of sugarcane wilt has primarily been done by replacing and/ or removing susceptible sugarcane varieties with new resistant varieties for cultivation. However, due to frequent breakdown of resistance, the varieties have to be replaced continuously. Use of fungicides has also not yielded desired results for wilt management besides being deleterious to environment and human health (Jahanshir and Dzhalilov, 2010).
In this scenario, use of biocontrol agents like Trichoderma offers a viable and ecofriendly option for disease management. Trichoderma spp. are cosmopolitan in occurrence. They are commonly found in all types of soil both as free living fungi as well as in endophytic association with crops (Bae et al., 2011; Harman et al., 2004). To date, these fungi have been explored extensively for management of several diseases in diverse crops (Sharma et al., 2014). In sugarcane, this fungus has been successfully explored for management of red rot of sugarcane caused by Colletotrichum falcatum Went (Joshi et al., 2016; Joshi et al., 2019). Besides, several strains of Trichoderma have been identified against Fusarium wilt of various other crops like eggplant (Solanum melongena L.) (Abdel-Monaim et al., 2014), chilli (Capsicum annum L.) (Bhat et al., 2016), tomato (Solanum lycopersicum L.) (Sundarmoorty and Balabaskar, 2013), etc.
In case of sugarcane, some preliminary studies with limited number of rhizospheric Trichoderma isolates have been conducted previously. Gawade et al. (2012) evaluated six Trichoderma isolates from rhizosphere soil against wilt of sugarcane and reported that all six strains showed antagonistic activity against F. moniliforme. The study also revealed that different isolates within same species showed different degree of inhibition. Similarly, Sabalpara et al. (2009) also assessed in vitro biocontrol potential of seven Trichoderma isolates against F. moniliforme, associated with sugarcane wilt and identified promising isolates. Similarly, Jena and Panigrahi (2017) also tested Trichoderma isolates against wilt in sugarcane. They identified different species of Trichoderma like T. viride, T. harzianum and T. pseudockei which showed antagonistic activity against F. moniliforme with T. viride exhibiting complete inhibition of F. moniliforme. However, in all these studies very limited number of Trichoderma isolates (six to seven only) have been evaluated for their antagonistic potential and the assessed isolates are restricted to those isolated from rhizospheric soils only. Moreover, in these studies Trichoderma isolates have been evaluated against F. moniliforme, as opposed to F. sacchari which has been recently established as the causal agent of sugarcane wilt based on molecular studies (Viswanathan et al., 2011).
In recent times, there has been major thrust on exploring various endophytic microbes including Trichoderma spp. for management of various plant diseases (Moghaddam and Soltani, 2013; Mulaw et al., 2013; Bailey et al., 2008). In the case of sugarcane wilt in particular, endophytic Trichoderma isolates can provide added advantage compared to rhizospheric isolates for disease management, since wilt is primarily sett borne in nature and as such, endophytic Trichoderma strains, which will be already adapted to the ecological niche where they have to function to suppress the plant pathogen i.e. within plant tissue, may be more successful as biocontrol agents as compared to rhizospheric isolates for wilt management. Also, evaluating a large number of isolates can increase chances of identifying highly potent antagonistic strains for further use.
Keeping these aspects in mind, the present study was undertaken to evaluate a substantive number of both rhizospheric and endophytic Trichoderma isolates against sugarcane wilt pathogen F. sacchari by different screening techniques in vitro and to identify potent isolates for further studies.
MATERIALS AND METHODS
Isolation and identification of Fusarium sacchari
For isolating Fusarium sacchari, the stalks of sugarcane variety Co 7717 showing symptoms of wilt were collected during October 2018 from the research farm of ICAR-Indian Institute of Sugarcane Research, Lucknow, India (GPS coordinates of the location: 26°56ʹ N, 80°52ʹ E and 111 m above mean sea level).
The collected stalks were washed with running tap water to remove adhering mud and soil particles and the infected canes were split open longitudinally with a sharp knife. The internal pith portion of internodes showing typical wilt symptoms was cut into small segments. The segments were surface disinfected with 95% ethanol for 30-45 seconds followed by washing thrice with sterile distilled water. The disinfected tissue was dried in a laminar flow on sterile filter paper and then plated on Petri dishes previously poured with Potato Dextrose Agar (PDA) (HiMedia) with three pieces per plate. The Petri dishes were incubated at 28±1°C for 7-8 days and monitored on routine basis for growth of the hyphae from the tissue margins. Growing hyphal tips were picked from typical colonies of Fusarium, and transferred to fresh PDA plates and maintained on PDA. These isolates produced abundant white mycelia on the PDA medium for three days. The colors of the colonies on both sides of the petri dishes were determined according to Summerell et al. (2003). Morphological characteristics of the fungus largely matched the descriptions in The Fusarium Laboratory Manual (Leslie and Summerell, 2006) Further molecular confirmation of the isolate was performed as per O’Donnell et al. (1998). Briefly, DNA was isolated from the 50 mg grown mycelium and Translation elongation factor 1-alpha (TEF1-α) gene was amplified using primer pairs of EF1/EF2 (O’Donnell et al., 1998). F. sacchari , colonies were purified on PDA medium and preserved at 4°C for further antagonistic studies.
Trichoderma isolates
One hundred and three Trichoderma isolates were used in the present study. Forty three of the selected isolates had been previously established from sugarcane rhizosphere soils collected from various geographical regions and farming systems (conventional/ organic) of sub-tropical India (Joshi and Misra, 2013; Joshi et al., 2019). In addition, 60 isolates of endophytic Trichoderma, established from root, stalk and leaf tissue of 10 different sugarcane varieties were also selected for the present study (Joshi et al., 2018). For further reference the rhizospheric isolates are designated as STr-1 to STr-126 while endophytic isolates are designated as SER1 to SER44 for root endophytes, SEL1 to SEL7 for leaf endophytes and SES1 to SES 13 for stalk endophytes. The source of the selected Trichoderma isolates is given in Table 1.
Evaluation of antagonistic activity of Trichoderma isolates
Screening by Dual Culture Method
Antagonistic effect of Trichoderma isolates against F. sacchari was evaluated using the dual culture technique (Dennis and Webster, 1971a). Briefly, 6 mm discs of the pathogen were cut from the edge of a 6-7 days old culture and placed at the edge of Petri dishes, previously poured with PDA.
Another disc cut from the edge of a 3-4 days old colony of test Trichoderma isolate was inoculated in the opposite direction of pathogen disc. The plates were incubated at 28±1 °C for 72 hours with three replications of each Trichoderma isolate.
Inoculated Petri plates with pathogen alone was maintained as a control. Radial growth of the pathogen was recorded after 72 h for both dual culture and control plates. The radial growth inhibition of pathogen by different isolates of Trichoderma was calculated as Inhibition % (I) by the formula: Inhibition % (I) =((R1-R2)/R1) × 100, where R1: Radial growth of pathogen in control and R2: Radial growth of pathogen in treatment.
Screening by Poison Food Technique
Based on results of Dual culture technique, superior isolates of endophytic Trichoderma were further evaluated for production of soluble inhibitory metabolites against Fusarium sacchari by using the poison food technique (Dennis and Webster, 1971b). Minimal salts broth was used as culture media for production of soluble inhibitory metabolites by Trichoderma (Srinivasan et al., 1992). Conical flasks (150 ml) with 100 ml minimal salts broth were inoculated with 1 ml of a spore suspension (106 cfu ml-1) of Trichoderma isolate prepared in sterile distilled water. These flasks were then incubated at 28 ± 1 °C.
After 20 days mycelium was removed by filtration using filter paper (Whatman No. 1) and the obtained culture filtrate was passed through a 0.22 μm membrane disposable filter unit (Whatman) for removal of spores. Culture filtrate obtained after filtration was added in potato dextrose agar (PDA) medium just before pouring in Petri plates to get a final concentration of 20% culture filtrate. After solidification a disc (5 mm) was cut from 5 days old culture of F. sacchari and inoculated in the center of the PDA poured plates. Control plates were amended with similar amount of un-inoculated minimal salts broth. For each isolate, four replications were maintained at 28 ± 1°C and Fusarium sp. isolate colony diameter after 7 days was recorded in all plates. Percent inhibition in growth was calculated as: Inhibition % (I) =((R1-R2)/R1) × 100, where R1: Radial growth of pathogen in control and R2: Radial growth of pathogen in treatment.
RESULTS AND DISCUSSION
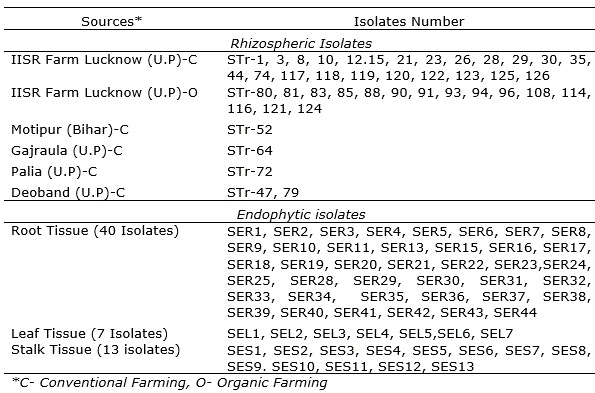
With regard to the macroscopic characteristics of the fungus, the color of the colony on PDA was initially orange-yellow and became purple-white when aged. On PDA, the growth was rapid with abundance of mycelia and the colony exhibited cottony hyphal growth (Figure 1 a, b). Colonies reached 2.3-3.7 cm in diameter at 25 °C and 2.1-3.4 cm at 30 °C after 3 days on PDA. Morphological characteristics of the isolated fungus were consistent with the description of Fusarium sacchari, which is a member of the Gibberella fujikuroi species complex (Leslie and Summerell, 2006). Abundant microconidia were observed in the aerial mycelium and were oval to ellipsoidal in shape and aseptate. Macroconidia, were slender or slightly falcate and three to five septate. The identification of F. sacchari was done based on the taxonomic guidelines by Leslie and Summerell (2006). For molecular identification of the putative Fusarium isolate, TEF1-α gene specific primer was used which resulted in an amplicon of 656 base pair size (O’Donnell et al., 1998).
Evaluation of antagonistic activity of Trichoderma isolates
The two evaluation techniques (dual culture and poison food technique) applied to assess the inhibitory potential of Trichoderma isolates on the growth of F. sacchari shown results (Table 2 and Table 3). In dual culture technique, considerable variability was observed in the inhibition of F. sacchari growth by the 103 Trichoderma isolates which ranged from a highest inhibition of 27.2% by isolate SER42 to lowest inhibition of 1.4% observed in isolate SER11 (Table 2, Figure 2).
On examining the inhibitory activity of the rhizospheric and endophytic Trichoderma isolates separately, it was observed that among the 43 rhizospheric isolates, the inhibitory percentage ranged between 1.6% (STr-64) to 26.1% (STr-29 & STr-80). Thirteen rhizospheric isolates exhibited inhibition in F. sacchari growth between the range of 20 to 30% while in 26 isolates inhibition was recorded in range of 10 to 20% and the remaining four isolates showed very poor inhibitory activity of < 10%.
In case of the 60 endophytic Trichoderma isolates, highest inhibition of 27.2% was recorded in isolate SER42 (a root endophyte). Thirty endophytic Trichoderma isolates (16 from roots, five from leaf and nine from stalk) showed inhibition ranging between 20 to 30% against F. sacchari while 19 isolates (13 from root, two from leaf and four from stalk) showed inhibition between 10 to 20%. The remaining 11 isolates (all isolated from root tissue) showed <10% inhibition in F. sacchari growth.
Overall, it was observed that endophytic isolates were more effective in suppressing F. sacchari growth than rhizospheric isolates as indicated by the fact that almost 50% (30 out of 60) of the endophytic isolates showed >20% inhibition in F. sacchari growth as compared to only 30% (13 out of 43 isolates) of the rhizospheric isolates showing >20% inhibition.

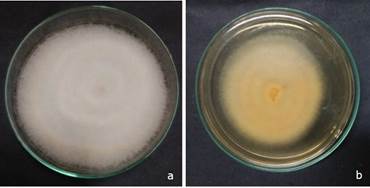
Figure 2. Antagonistic activity of endophytic Trichoderma against Fusarim sacchari in dual culture studies, (a) Control Plate, (b) Trichoderma SER 32 isolate showing antagonistic activity, (c) Overgrowth of Trichoderma over Fusarium.
Table 2. Antagonistic activity of rhizospheric and endophytic Trichoderma isolates against Fusarium sacchari in dual culture assay.

Since in dual culture studies endophytic isolates were observed to show higher inhibitory activity against F. sacchari than rhizospheric isolates, 12 endophytic isolates (five from roots, two isolates from leave and five isolates from stalk) were selected for further studies and examined for production of soluble inhibitory metabolite against F. sacchari .
All the selected 12 isolates had exhibited >20% inhibition in F. sacchari growth in dual culture studies (Table 3, Figure 3). The results of the study revealed significant variability across the 12 isolates. The inhibition percentage varied from 1.4% (SER 43) to 44.2% (SER10). Eight out of the 12 isolates showed inhibition percentage less than 11% while three isolates (SER15, SER42, SES12) exhibited inhibition between 20% to 29.9% and one isolate (SER10) showed highest inhibition of 44.2%. Overall, four of the 12 isolates showed >20% inhibition in F. sacchari growth in poison food technique (Table 3, Figure 3).
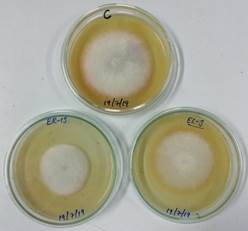
Figure 3. Inhibition of Fusarium sacchari by soluble inhibitory metabolites of endophytic Trichoderma isolates ER13 and El-3. C-control
Table 3. Inhibition of Fusarium sacchari by diffusible secondary metabolite produced by endophytic Trichoderma isolates by Poison Food Technique.

Overall, the results of the dual culture and poison food assay of the Trichoderma isolates revealed considerable variability in the inhibitory potential of the isolates in both studies. Several previous workers have also reported varying levels of inhibition of Fusarium spp. by both endophytic and rhizospheric strains of Trichoderma. Both, Sabalpara et al. (2009) and Gawade et al. (2012) had in vitro evaluated Trichoderma isolates against F. moniliforme and observed variability in antagonistic activity among Trichoderma isolates. Dolatabadi et al. (2012) reported variability in suppression of Fusarium spp. causing wilt of lentil by root endophytic strains of Trichoderma . Similarly, Taribuka et al. (2017) reported variability in inhibitory activity of endophytic Trichoderma isolates against Fusarium causing banana wilt.
On the basis of the results of dual culture and poison food assay, isolate SER10 was identified as the most potent isolate in the study exhibiting high inhibition in F. sacchari growth in both the assays. Trichoderma species are well documented biological control agents which have been previously explored for management of wilt caused by Fusarium spp. in various crops. Sundarmoorthy and Balabaskar (2013) evaluated the efficiency of Trichoderma against F. oxysporum, causing wilt disease in tomato (Solanum lycopersicum L.) and observed it to be effective. Similarly, Adhikary et al. (2017) stated that pre inoculation application of Trichoderma significantly reduced the severity of the wilt disease in eggplant (Solanum melongena L.). Anuragi and Sharma (2016) evaluated Trichoderma isolates in vitro against F. oxysporum, causal agent of wilt disease in chickpea and observed that T. reesei showed the strongest antagonistic activity against Fusarium in dual cultured followed by T. viride and T. harzianum.
However, despite there being a number of studies exploring potential of Trichoderma in other crops, there have been very limited studies on evaluating Trichoderma against sugarcane wilt. A few of these previous studies, the focus has been on evaluating antagonistic potential of six to seven Trichoderma isolates against Fusarium sp. in vitro and potent isolates were identified (Sabalpara et al., 2009; Gawade et al., 2012). The major limitation with these studies has been that they have been conducted using a very small number of rhizospheric isolates (six-seven) only and moreover, the isolates were evaluated against F. moniliforme, as opposed to F. sacchari which is now recognized as the causal agent of sugarcane wilt. Since, sugarcane wilt is primarily a set borne disease, the use of potent endophytic Trichoderma strains can give added advantage as compared to rhizospheric strains in managing this disease. The endophytic strains will be better adapted to establish and inhibit target pathogen within plant tissue as compared to rhizospheric strains which may function better in soil and plant rhizosphere. Several endophytic Trichoderma spp. has been used against Fusarium spp. causing wilt in crops other than sugarcane. For instance, Dolatabadi et al. (2012), reported suppression of Fusarium spp. causing wilt of lentil (Lens culinaris Medikus) by root endophytic strains of Trichoderma . Similarly, Taribuka et al. (2017) evaluated endophytic Trichoderma isolates against Fusarium spp. causal agent of wilt disease in banana (Musa spp.).
However, this is the first study reporting evaluation of the antagonistic effect of substantive number (103) of rhizospheric as well as endophytic Trichoderma isolates against F. sacchari causing sugarcane wilt and identification of promising isolates. Moreover, we also observed that, in dual culture assays, the endophytic isolates were more effective in suppressing F. sacchari growth than rhizospheric isolates as indicated by the fact that almost 50% (30 out of 60) of the endophytic isolates showed >20% inhibition in F. sacchari growth as compared to only 30% (13 out of 43 isolates) of the rhizospheric isolates showing >20% inhibition.
As such, the potent endophytic Trichoderma isolates established from this study can provide advantage in case of sugarcane diseases management since endophytic strains will be better adapted to establish and inhibit target pathogen within plant tissue as compared to rhizospheric strains which may function better against soil borne pathogens. Based on the results of both the assays, Trichoderma isolate SER 10, isolated from root tissue of sugarcane, was found most promising showing 44.2% inhibition of Fusarium growth in poison food method and 26.3% inhibition in dual culture studies. However, further studies on field evaluation of this isolate for wilt management are essential to establish their efficacy and their further exploitation.
CONCLUSIONS
The present evaluation of study conducted using 103 Trichoderma isolates, representing both rhizospheric as well as endophytic isolates, revealed that in general, endophytic Trichoderma strains show higher potency in suppressing F. sacchari growth as compared to rhizospheric isolates. A potent endophytic Trichoderma SER10 strain show high inhibitory activity against wilt pathogen. The isolate needs to be explored further for wilt management in field.