Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cuban Journal of Agricultural Science
versión impresa ISSN 0864-0408versión On-line ISSN 2079-3480
Cuban J. Agric. Sci. vol.49 no.3 Mayabeque jul.-set. 2015
ORIGINAL ARTICLE
Increasing doses of a microbial preparation in the health and productive performance of post-weaning pigs
Dosis crecientes de un preparado microbiano en el comportamiento productivo y de salud de los cerdos en posdestete
L. Flores,I A. Elías,II Yolaine Medina,II F. Proaño,I G. Granizo,I Sandra López,I W. Caicedo,III
IEscuela Superior Politécnica de Chimborazo, Río Bamba, Ecuador.
IIInstituto de Ciencia Animal, Apartado Postal 24, San José de las Lajas, Mayabeque, Cuba.
IIIUniversidad Estatal Amazónica, km 2 ½ Vía a Napo, Pastaza, Ecuador.
ABSTRACT
In order to evaluate growing doses of a microbial preparation (milk whey 33 %, molasses 20 %, urea 1 %, mineral salt 1 %, and water 45 %) on the productive performance, and the presence of diarrheas in post-weaning pigs, a completely randomized design was applied, with five treatments and four repetitions: T1 concentrate, T2, T3, T4 (concentrate plus 5 mL kg LW-1, 10 mL kg LW-1, and 15 mL kg LW-1 of the microbial preparation, respectively) and T5 (concentrate plus commercial probiotic). A total of 200 pigs from the crossing of Landrace x Large White with Belgium White x Pietrain were used, with 28 d old and 6.99 kg LW± 0.18 kg. The highest final weight and the best total and daily weight gain (P < 0.01) were obtained in pigs fed with concentrates, and 15 mL kg LW-1 of the microbial preparation was added. Values of 25.78 kg, 18.78 kg and 447.25 g were obtained, respectively. The most efficient values, according to the conversions of dry matter, protein and energy (P < 0.01), were obtained in the treatment with 15 mL.kg LW-1 of microbial preparation, with 1.68 kg DM.kg-1, 351.16 g.kg LW-1, and 24.54 MJ.kg LW-1, respectively. The lowest presence of diarrheas (P < 0.01) was obtained in animals fed with the concentrate plus 15 mL kg LW-1 of the microbial preparation, and an incidence of 12 diarrheas. The use of the microbial preparation (15 mL kg LW-1) improved the nutritional value of the concentrates, the best parameters were obtained and the appearance of diarrheas was reduced.
Key words: probiotic, nutritional quality, pigs, digestibility.
RESUMEN
Para evaluar dosis crecientes de un preparado microbiano (suero de leche 33 %, melaza 20 %, urea 1 %, sal mineral 1 %, agua 45 %) y un probiótico comercial ML 100 E (1 kg/t) en el comportamiento productivo y presencia de diarreas en cerdos durante la etapa postdestete, se aplicó un diseño completamente aleatorizado, con cinco tratamientos y cuatro repeticiones: T1 concentrado, T2, T3, T4 (concentrado más 5 mL.kg PV-1, 10 mL.kg PV-1,15 mL.kg PV-1 del preparado microbiano, respectivamente) y T5 (concentrado más probiótico comercial). Se utilizaron 200 cerdos del cruce Landrace x Large White con Blanco Belga x Pietrain, de 28 d de edad, con 6.99 kg PV ± 0.18 kg. El mayor peso final y la mejor ganancia de peso total y diaria (P < 0.01) se obtuvieron en los cerdos alimentados con concentrado, al que se agregó15 mL.kg PV-1 del preparado microbiano. Se lograron valores de 25.78 kg, 18.78 kg y 447.25 g, respectivamente. Los valores más eficientes, en cuanto a la conversiones de materia seca, proteína y energía (P < 0.01), se obtuvieron con el tratamiento al que se añadió el preparado microbiano (15 mL.kg PV-1), con cifras de 1.68 kg MS.kg-1 y aumento de peso vivo de 351.16 g.kg PV-1 y 24.54 MJ.kg PV-1, respectivamente. La menor presencia de diarreas (P < 0.01) se registró en los animales alimentados con concentrado más 15 mL.kg PV-1 de preparado microbiano, con incidencia de 12 diarreas. La utilización del preparado microbiano (15 mL.kg PV-1) mejoró el valor nutricional de los concentrados. Se obtuvieron además, los mejores parámetros productivos y se redujo la ocurrencia de diarreas en cerdos posdestete.
Palabras clave: probiótico, calidad nutricional, cerdos, digestibilidad.
INTRODUCTION
The need of controlling digestive and respiratory pathologies, in the intensive systems of pig production, has led to the wide use of antibiotics as food additives (Cajarville et al. 2011). However, the utilization of low doses of antimicrobial products for feeding animals destined to human consumption, in order to improve growth and prevent diseases, is related to the global crisis of health regarding the resistance to antimicrobials.
At international level, several jurisdictions have restricted the use of these products (Maron et al. 2013). The use of probiotics, as live microbial additives, is an alternative that provides benefits to animal health because it improves the intestinal microbial balance. The application of probiotics on pig feeding has allowed to improve the zootechnical indicators of food conversion, final liveweight gain and immune response (Jurado et al. 2013).
According to Gutiérrez et al. (2013), most of the authors coincide to define probiotics as food additives formed by living microorganisms that, consumed in proper amounts, may produce a beneficial effect on physiology and health of the host, improving the intestinal microbial balance.
Elías and Herrera (2008) reported on the obtaining and use of a product with probiotic activity, result of a simple biotechnological process, which is rich of lactobacilli, yeasts, organic acids of carbonated short chains, and low pH, able to control the development of Escherichia coli, to reduce the incidence of diarrheas and to increase the liveweight gain of animals.
In Ecuador, some microbial preparations with excellent probiotic activity have been used, which are based on whey, sugarcane juice and pig feces as bio-accelerant. These last improve the quality of bio-preparations because they are rich in organic acids, lactic acids and yeasts (Díaz 2011).
The objective of this research was to perform a chemical and microbiological characterization of the microbial preparation, compare the nutritional content of experimental diets, and evaluate the productive performance and diarrheas during the post-weaning stage of pigs.
MATERIALS AND METHODS
Experiment location. The experiment was developed in the labs of animal nutrition and biotechnology at the Unidad Académica Porcina in the Facultad de Ciencias Pecuarias (FCP) from the Escuela Superior Politécnica de Chimborazo, Ecuador (ESPOCH). The experiment was divided into three stages:
First stage. Production and characterization of the microbial preparation. This stage lasted 9d. The fresh whey, molasses, urea, mineral salt and water were mixed (table 1) in plastic tanks (220 L of volume), according to recommendations of Díaz et al. (2013).
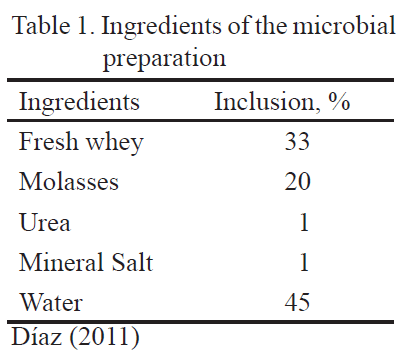
The whey was taken from the industrial cheese factory from the FCP, ESPOCH. The mixture contained 85º Brix. Urea was commercially acquired and contained 46 % of nitrogen. The mineral salt was also commercially acquired and was composed by 9 % of calcium and 10 % of phosphorous. The water for human consumption was used after two hours of rest. The components of the mixture were homogenized and contained with a hermetic top at room temperature.
The mixture was homogenized and with a top for 96 h. Five samples (200 mL) were taken for characterizing the product, which consisted on determining the pH (WPA portable potentiometer), the content of organic acids according to the technique proposed by Erwin et al. (1961) and microorganism recount (lactic bacteria, fungi and yeasts) according to the procedure proposed by Merck (2005).
The descriptive statistics from Infostat (2012) was used to characterize the microbial preparation.
Second stage. Comparison of the nutritional content of experimental diets. This stage lasted
30 d. A concentrate was formulated and considered as base diet (table 2 and 3), and contained the same raw matters for the post-weaning stage due to the nutritional requirements of the animals, according to NRC (1998).
In order to estimate the nutritional influence that could result from the addition of the microbial preparation of Díaz et al. (2013) over the addition of a probiotic cited by Allen et al. (2013), a lab analysis was performed for five treatments: base concentrate, base concentrate + microbial preparation (5 mL kg LW-1), base concentrate + microbial preparation (10 mL kg LW-1), and base concentrate + microbial preparation (15 mL kg LW-1) and base concentrate + commercial probiotic (ML 100 E).
The commercial probiotic (More Yeast 100 E) is a combination of yeast culture with live cells of Saccharomyces cerevisiae, Bacillus subtilis and digestive enzymes (protease, lipase, amylase and cellulase).
All treatments underwent a proximal analysis, according to AOAC (2005). In vitro digestibility of crude protein was determined by the technique of pepsin and pancreatin (Dierick et al. 1985) and true protein was determined according to Bernstein (1983).
For comparing the nutritional content of the experimental diets, the means of each treatment were used after analyzing five samples.
Third stage. Evaluation of productive indexes in fattening pigs. The initial weight, final weight, total weight gain, daily weight gain, and the conversion of dry matter, protein and energy were evaluated, regarding the same treatments of second stage.
Experimental procedure. An amount of 200 castrated Landrace-Large White x Belgium White-Pietrain pigs, with 28d old and 6.99 ± 0.18 kg, were used. Each experimental unit was composed by 10 pigs per collective pens of 2 x 2.25 m, with a density of a pig per 0.45 m2. The animals received five experimental treatments. The food was provided every 24h during the morning, at 8:00 a.m. water was provided ad libitum in nipple troughs.
Statistical analysis. For the productive performance, an analysis of covariance was carried out in the variables final weight, total weight gain, daily weight gain and conversion of dry matter, protein and energy. The initial weight was considered as the concomitant variable, which did not influence on the mentioned variables so the analysis of variance, according to a completely randomized design, was carried out (InfoStat 2012), with five treatments and four repetitions per treatments. The test of Duncan (1955) was applied (P < 0.05) in the necessary cases.
The theoretical assumptions of the analysis of variance for the amount of diarrheas were analyzed. The test of Shapiro and Wilk (1965) was used for normality of errors. For homogeneity of variance, the test of Levene (1960) fulfilled these assumptions, so it was not necessary its transformation (√x) through the statistical software StatSoft, Inc. (2003).
RESULTS AND DISCUSSION
The microbial preparation showed 3.8 of pH after 96 h of fermentation. The content of lactic acid was 0.122mg mL-1; the propionic acid was 0.00367 mg mL-1, and the butyric acid was 0.00037 mg mL-1. The acidity considered as lactic acid was 3.26 %, 21.23 % of dry matter, 1.383 % of total nitrogen, 0.953 % of protein content, 0.184 % of ammonia content and 8.62 % of crude protein.
There was a total absence of salmonellas and coliforms. The content of mold and yeasts was 384 x 103 CFU mL-1, 8.90 ºBrix and that of acid lactic bacteria was 43.12 x 103 CFU mL-1.
After comparing the microbial preparation with Vitafert (Elías and Herrera 2008), there were some similarities regarding their microbiological and chemical composition, because the content of yeast of Vitafert ranged between 107 and 108 CFU mL-1. Lactobacilli were between 109 and 1010 CFU mL-1, lactic acid ranged between 40.5 and 54.04 mg mL-1, and the acetic acid was between 13.51 and 25.82 mg.mL-1 (Roján 2009).
Table 4 shows the results of the proximal analysis, true protein and digestibility of crude protein from the five diets under study.
It is evident that there was a direct relationship between the addition of the microbial preparation and humidity. Regarding the dry matter, while the inclusion level of the microbial preparation increased in the concentrate, the dry matter decreased. The control showed the highest content, followed by the commercial probiotic without the microbial preparation, possibly because of the hydrolytic activity of acid lactic bacteria and yeasts, with the production of CO2 and H2O, as other researchers have reported regarding different food (Aksu et al. 2004, Nkosi 2009 and Weinberg et al. 2009).
The highest values of ash content were obtained with the inclusion of 5 and 10 mL.kg LW-1, followed by the inclusion of 15 mL.kg LW-1 and the control. The commercial probiotic without the microbial additive had the lowest content. It was demonstrated that as the amount of microbial preparation in the concentrate increased, the content of dry matter decreased and the ash concentration increased.
It is possible that the decrease of dry matter with the increase of the amount of microbial preparation may be caused by the low content of dry matter in this preparation, because it only contained 8.90 ºBrix. It is also possible the appearance of a solid state fermentation (SSF) during the time between the addition of the preparation, taking of the sample and the physical and chemical analysis because the NFE decreased with the increase of the amount of microbial preparation, with the lowest values in the addition of 15 mL.kg LW-1.
The decrease of NFE could not be caused by the accelerate growth of bacteria and yeasts added with the microbial preparation, which may have developed during the process of SSF. A similar effect was observed by Elías et al. (1990) through the SSF of sugar cane for obtaining a food for animals called Saccharina. Likewise, Elías and Herrera (2008) confirmed a similar effect in the production of other food.
The reduction of NFE was directly related to the increase of crude protein (table 4), regarding the increase of the amount of microbial preparation in the concentrates. Likewise, there was an increase of true protein. Something similar happened to a preparation of lactic acid bacteria in mixed diets of alfalfa or maize stubble plus a commercial concentrate after promoting the synthesis of microbial protein (Franco et al. 2009). In this regard, Elías et al. (1990) demonstrated that the efficiency for CP synthesis in the conversion of soluble carbohydrates within the NFE was 0.61 units.
Table 4 shows that control, the concentrate with the addition of 15 mL.kg LW-1 and that with the commercial probiotic had the highest fat content. The difference in the fat content among concentrates is not considered as significant because, according to NRC (1998), there are no differences in the performance of post-weaning pigs, fed with concentrates containing between 2 and 32 % of fat. These concentrates had the lowest content of crude fiber, with a little difference among them, because the crude fiber content of concentrates is considered low (6.31-8.3) and it is under the 10-15 % of the diet. The intake and the performance of animals are not affected by these differences, according to NRC (1998).
Regarding the variation in the digestibility of crude protein obtained, table 4 shows its increase compared to the control, between 6.29 and 6.95 percentile units. There were almost no differences among the inclusion levels of the microbial preparations but it was slightly superior to the increase obtained with the commercial probiotic, which increased 5.58 percentile units regarding the control.
It is known that lactic acid bacteria have a great proteolytic activity (Kunji et al. 1996). Regarding reports of Díaz et al. (2013), this microbial preparation also has this characteristic and a high content of lactic acid bacteria that justified the increase of the digestibility of the obtained CP. Something similar occurred with the addition of the commercial probiotic due to the action of live bacteria and yeasts and the enzymatic complex (protease, lipase, amylase and cellulase).
Table 5 shows the results of final weight obtained during the experiment. There were significant differences (P < 0.05) among treatments. The treatment with the addition of 15 mL.kg LW-1 of the microbial preparation had a difference over 5.66 kg of LW compared to the control. Regarding the inclusion of 5 mL.kg LW-1, the difference was 4.56 kg. With the addition of 10 mL.kg LW-1, the difference was 1.84 kg and there were 2.11 kg with the commercial probiotic.
As for total weight gain, there were significant differences (P < 0.05) among treatments. The treatment with 15 mL.kg LW-1 provided figures superior to 5.63, 4.54, 1.85 and 2.11 kg, regarding the control and the treatments with the inclusion of 5mL.kg LW-1, 10 mL.kg LW-1 and the commercial probiotic, respectively.
There were significant differences (P < 0.05) among treatments in daily weight gain. The treatment with 15 mL.kg LW-1 provided figures superior to 134.25, 108.25, 44.25 and 50.5 g, regarding the control, 5mL.kg LW-1, 10 mL.kg LW-1 and the commercial probiotic, respectively.
There was no statistical analysis for the variables dry matter intake, protein intake and energy intake per day because there was no variability within treatments, only the means of each treatment were reported. Dry matter intake increased with the increase of the amount of the product added to the concentrate. The intake of protein and energy also increased when the animals consumed higher quantities of dry matter. The amount of protein and energy was superior in the treatments where higher amounts of the microbial preparation were added.
Table 5 demonstrates that the conversion of dry matter, crude protein and digestible energy was higher with the increase of the amount of microbial preparation. The best conversions were achieved with the inclusion of 15 mL.kg LW-1 and with the commercial probiotic.
All the obtained productive indicators were more efficient than those recommended by NRC (1998) in this category and final liveweight. Likewise, these indicators coincide with the Cuban technical productive indicators referred by López et al. (2008). Weight gains obtained with higher inclusion levels of the microbial preparations agree with that recommended by the Grupo Porcino (GRUPOR 2010) in systems of intensive rearing with the use of concentrate food.
Cortés and Gómez (2011) determined that the use of probiotics in diets for pigs during their first life stages have a beneficial effect on the improvement of nutrient absorption in the small intestine. This is demonstrated on the improvement of zootechnical paramenters and on the decrease of diarrheas. Giang et al. (2012) improved the productive indicators in post-weaning pigs with the supplementation of diets with a complex of lactic acid bacteria, alone or combined with B. subtilis and S. boulardii.
Table 6 shows the number of diarrheas during the 43 d of study. There were significant differences (P < 0.05) among treatments, with the lowest value for the treatment with 15 mL.kg LW-1.
These values coincide with those reported by Jurado et al. (2013), who researched the in vivo evaluation of Lactobacillus plantarum, as an alternative for the use of antibiotics in piglets. Thirabunyanon and Thongwittaya (2012) reported that a probiotic strain of B. subtilis (NC11) has a protective action against the infection by S. enteritidis, and it is capable of excluding this last from its place in the gastrointestinal tract where the flow of pathogenic food starts. Heo et al. (2012) stated that probiotics and prebiotics seem to be able of improving yield and enteric health of piglets.
It is possible that the results of this study are directly related to the beneficial changes produced in the intestinal microbial composition, favoring the implantation of acid lactic bacteria, which, by their metabolic activity, control the proliferation of undesirable enterobacteria, and increase the efficiency of utilization of nutrients that are not digested by the enzymatic system of animals, in addition to increase the activity of the immune system, as the review by Metges and Loh (2003) demonstrates.
The resukts obtained during the experimental period agree with other researches on the use of bacteria cultures. Rodríguez (2009) obtained the best results in growing pigs, using a biological preparation of Lactobacillus acidophillus and Streptococcus termophillus. Pérez (2008) considered that including a mixed strain of yogurt (Lactobacillus bulgaricus/Streptococus thermophylus) for piglets under conditions of commercial production improved the productive indicators of pigs at 70 d old. Roján (2009) found a better response in the evaluation of the effect of a biologically active product (Vitafert) on health and productive indicators in pre-fattening pigs.
It can be concluded that the microbial preparation maintains a low pH because it does not contains short chain organic acids, lactic acid bacteria and yeasts. This guarantees its quality and stability, improves the nutritional value of concentrates, increases digestibility of crude protein, favors the productive parameters and reduces the indexes of diarrheas in pigs during the post-weaning stage. From the results of this research, new studies are recommended with the use of 15 mL.kg LW-1 of the microbial preparation. Its comparison with commercial antibiotics and probiotics is also suggested.
REFERENCES
Aksu, T., Baytok, E. & Bolat, D. 2004. ‘‘Effects of a bacterial silage inoculant on corn silage fermentation and nutrient digestibility’’. Small Ruminant Res., 55: 249.
Allen, H., Levine, U., Looft, T., Bandrick, M. & Casey, T. 2013. ‘‘Treatment, promotion, commotion: antibiotic alternatives in food-producing animals’’. Trends Microbiology, 21: 114.
AOAC 2005. Official Methods of Analysis. 18th ed., Gaithersburg, MD, USA: Assoc. Off. Agric. Anal. Chem. Inc.
Bernstein, L. 1983. Análisis de alimento. Wintra A. L. & Wintro K. B. (eds.), vol. 1, Pueblo y Educación, 84 p.
Cajaeville, C., Brambillasca, S. & Zumino, P. 2011. Revista Porcicultura Iberoamericana, 1: 2.
Cortés, L. & Gómez, F. 2011. ‘‘Eficiencia de microorganismos (EM) en el mejoramiento del sistema digestivo de cerdos en fase pre levante’’. Spei Domus., 7: 31.
Díaz, B. 2011. ‘‘Aprovechamiento biotecnológico de residuos agroindustriales para alimentación de animales zootécnicos’’. Rev. Colombiana de Ciencias Pecuarias, 4: 145.
Díaz, B. 2013. ‘‘Nutritional and economical efficiency of three biosilages from agroindustrial wastes in beef cattle’’. Cuban Journal of Agricultural Science, 47: 143.
Dierick, M. A., Decuypere, J. & Henderichx, H. 1985. ‘‘Protein digestion in pig measured in vivo’’. In: Just A., Jorgensen H. & Fernández J. (eds.), Proceedings of the 3rd International Seminar on Digestive Physiology in the Pig, pp. 239–332.
Di Rienzo, J. A., Casanoves, F., Balzarini, M. G., González, L., Tablada, M. & Robledo, C. W. 2012. InfoStat. version 2012, (Windows), Universidad Nacional de Córdoba, Argentina: Grupo InfoStat, Available: <http://www.infostat.com.ar/> .
Duncan, D. B. 1955. ‘‘Multiple range and multiple F tests’’. Biometrics, 11: 1.
ECUAQUIMICA 2000. Pecutrin® saborizado. Minerales + Vitaminas A, D3, E. Suplemento mineral más vitaminas ADE. no. 1AB-630-AGROCALIDAD, Available: <http://www.ecuaquimica.com/pdf_ganaderia/Pecutrin.pdf>, [Consulted: February 1, 2015].
Elías, A. & Herrera, F. 2008. Producción de alimentos para animales a través de procesos biotecnológicos sencillos con el empleo de microorganismos beneficiosos activados (MEBA). Vitafer. La Habana, Cuba: Instituto de Ciencia Animal.
Elías, A., Lezcano, O., Lezcano, P., Cordero, J. & Quintana, L. 1990. ‘‘A review on the development of a protein sugar cane enrichment technology through solid state fermentation (Saccharina)’’. Cuban Journal of Agricultural Science, 24: 1.
Erwin, E., Marco, G. & Emery, E. 1961. ‘‘Volatile fatty acid analysis of blood and rumen fluid by gas chromatography’’. J. Dairy Sci., 44: 1788.
Franco, J., Galina, N., Delgadillo, P. & Pérez-Gil, F. 2009. ‘‘Efecto de la adición de un suplemento liquido portador de bacterias acido lácticas a dietas de alfalfa-concentrado y rastrojo de maíz-concentrado en ovinos’’. Asociación Latinoamericana de Producción Animal, 17: 83.
Giang, H., Viet, T., Ogle, B. & Lindberg, J. 2012. ‘‘Effects of supplementation of probiotics on the performance, nutrient digestibility and faecal microflora in growing-finishing pigs’’. Asian-Aust. J. Anim. Sci., 24: 665.
Grupor 2010. Boletín anual de indicadores productivos en la producción porcina en cuba. MINAG, 32 p.
Gutiérrez, L., Montoya, O. & Vélez, J. 2013. ‘‘Probióticos: Una alternativa de producción limpia y de reemplazo a los antibióticos promotores de crecimiento en la alimentación animal’’. Producción + limpia, 8: 135.
Heo, J., Opapeju, F., Pluske, J., Kim, J., Hampson, D. & Nyachoti, C. 2012. ‘‘Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhea without using in-feed antimicrobial compounds’’. J. Animal Physiology and Animal Nutrition, 97: 207.
Jurado, H., Ramírez, C. & Martínez, J. 2013. ‘‘Evaluación in vivo de Lactobacillus plantarum como alternativa al uso de antibióticos en lechones’’. MVZ Córdoba, 18: 3649.
Kunji, E. R. S., Mieran, J., Hagting, A., Poulamn, B. & Konings, W. N. 1996. ‘‘The proteolytic systems of lactic acid bacteria’’. Antonie van Leewoenhock, 70: 221.
Levene, H. 1960. Robust tests for the equality of variance. Contributions to Probability and Statistics. Stanford University Press, 278-292 p.
López, O., Pérez, J., García, A., Dieguez, F., Sosa, R. & Crespo, A. 2008. Manual de procedimientos técnicos para la crianza porcina. La Habana, Cuba: Ministerio de Agricultura, Ediciones CIMA, 136 p.
Maron, D., Smith, T. & Nachman, K. 2013. ‘‘Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey’’. Globalization and Health, 9: 48.
Merk 2005. Catálogo de productos, técnicas y servicios en medios de cultivo para microbiología. Alemania, 45-49 p.
Metges, C. & Loh, G. 2003. Intestinal microbial amino acid synthesis and its importance for the amino acid homeostasis of the monogastric host. Progressing research on energy and protein metabolism. (ser. EAAP Publication, no. ser. 109), Sonffrand, C.C Metges, 579-992 p.
Nkosi, B. D., Meeske, R., Palic, D., Langa, T., Leeuro, K. J. & Gtoenewald, J. B. 2009. ‘‘Effects of ensiling whole crop maize with bacterial inoculants on the fermentation, aerobic stability, and growth performance of lambs’’. Anim. Feed. Sci. Tech., 154: 193.
NRC 1998. Nutrient Requirement of Domestic Animal. Nutrient Requirement of swine. Washington D.C.: Nat. Acad. Sci., 85-90 p.
Peréz, Y. 2008. ‘‘Evaluación del efecto probiótico de una cepa mixta de yogurt (Lactobacillus bulgaricus/ Streptoccocus thermophylus) para cerditos en condiciones de producción porcina comercial’’. Rev. Computarizada de Producción Porcina, 15: 4.
Rodriguez, J., Carmenate, M., Hernández, J., Guerra, A., Calero, I., Álvarez, J., Martín, E. & Suárez, M. 2009. ‘‘Evaluación del suministro de un preparado biológico de Lactobacillus acidophillus y Streptococcus termophillus en cerdos en crecimiento’’. Revista Computadorizada de Producción Porcina, 16: 54.
Roján, L. 2009. Efecto de un producto biológicamente activo (Vitafer) en indicadores productivos y de salud en preceba porcina. M.Sc. Thesis, Instituto de Ciencia Animal, La Habana, Cuba.
Shapiro, S. & Wilk, B. 1965. ‘‘An analysis of variance test for normality (complete samples)’’. Biometrika, 52: 602.
StatSoft Inc. 2003. STATISTICA (data analysis software system). version 7.
Thirabunyanon, M. & Thonwittaya, M. 2012. ‘‘Protection activity of a novel probiotic strain of Bacillus subtilis against Salmonell enteritubis infection’’. Research Vet. Sci., 97: 74.
Weinberg, Z., Shatz, O., Chen, Y., Yosef, E., Nikbahat, M., Ben Ghedalia, D. & Miron, J. 2009. ‘‘Effect of lactic acid bacteria inoculants on in vitro digestibility of wheat and corn silages’’. J. Dairy Sci., 90: 4754.
Received: April 3, 2014
Accepted: March 23, 2015
L. Flores, Escuela Superior Politécnica de Chimborazo, Río Bamba, Ecuador. Email: luisgerardofloresmancheno@yahoo.es














