Lactic acid bacteria, mainly those of Lactobacillus genus, are among the microorganisms that are more studied and used as probiotic candidates or are included in probiotic products and functional feeds (Saura-Calixto and Goñi 2005 and Boyle et al. 2006). Strains of Streptococcus, Enterococcus, Pediococcus, Leuconostoc, Bacillus, Bifidobacterium, Propionibacterium, Escherichia coli (non- pathogenic strains), Aspergillus and yeasts (Bajagai et al. 2016) are also used.
García-Hernández et al. (2016) isolated, identified and characterized lactic bacteria, of bird orgin, as probiotic candidate. These authors found that Lactobacillus pentosus LB-31 was the most promising strain and evaluated its potentialities in an essay with broilers. However, other identified strains in the study, like Pediococcus pentosaceus (LB-12 and LB-25), could be evaluated or included in multispecies probiotics, that encourage the beneficial effects of cultures of individual strains, as well as symbiotic formulations (Bomba et al. 2002 and Bajagai et al. 2016).
Bacteria of Pediococcus genus are presented in the shape of coccus (tetrads) and are anaerobial, Gram positive, not mobile, non-sporulated, facultative and negative catalase (Porto et al. 2017). According to these authors, Pediococcus pentosaceus and Pediococcus acidilactici are the main species used in probiotic supplements for animals and humans, as well as in the production of bacteriocins (pediocin) and inoculum in fermentative processes to avoid contaminations. Nevertheless, to be considered as probiotics, they must fulfill the requirements published by FAO and WHO (2002), as well as demonstrate their efficiency, which depends on the strain or strains used, among other factors (Yang et al. 2009 and Endo and Gueimonde 2016).
Among the in vitro characteristics to be evaluated, generally, for selecting probiotic strains, are the tolerance to low pH, high concentrations of biliary salts, growth ability of candidates, antimicrobial activity and susceptibility to antimicrobials. Determination of these last characteristics is the objective of this study, with the evaluation of two strains of Pediococcus pentosaceus (LB-12 y LB-25) and their growth ability with the use of fructans of Agave fourcroydes (García-Curbelo et al. 2015a, 2015b) as energy source in a cultivation medium, which could favor the generation of symbiotic futures.
Materials and Methods
Four trials were conducted for studying growth dynamics of lactic bacteria strains in a MRS (Man, Rogosa and Sharpe) medium, their growth ability in the presence of fructans of Agave fourcroydes (FRUCTOICA) as energy source of the medium and their antimicrobial activity and susceptibility to antimicrobials.
Bacteria characteristics. Strains used belonged to Pediococcus pentosaceus LB-12 and LB-25, of bird origin, from the Banco de Microorganismos para la Producción Animal (BAMIPA) of the Instituto de Ciencia Animal (Mayabeque, Cuba). These strains were identified by sequencing of ribosomal RNA 16S gen and its sequences are located in the GenBank (Access number: FR717460 and FR717465). In addition, they were previously characterized as probiotic candidates according to their growth ability at 24 h, lactic acid production, tolerance to acid pH and high concentrations of biliary salts(García-Hernández et al. 2016). In this study, both strains of lactic bacteria, preserved in a MRS medium with glycerol at 15 % (v/v) and freezing at -80 ºC, were activated with two sub-cultures in MRS medium (Oxoid,UK) at 37 ºC from 18 to24 h. From the active culture, a suspension of each strain (LB-12 and LB-25) was prepared in a MRS medium (pH 6.20±0.2) with optical density (OD) at 600 nm of 0.125 equivalent to 107 cfu•mL-1. Inocula concentration was verified through culture in agar MRS plates (Oxoid, UK).
Growth dynamics of P. pentosaceus in MRS medium. An amount of 20 µL of each suspension were inoculated in cells of the microplate, that contained MRS medium (1/10, v/v). Growth was monitored in a Microplate Spectrophotometer System SpectraMax 340 (Molecular Devices, USA). Each cell was considered an experimental unit. Optical density (OD) at 600 nm was measured every 30 min. per 12 h of incubation at 37 ºC. It was verified the failure in the fulfillment of normality assumption by Shapiro and Wilk (1965) test for this variable, which distribution was fitted to Gamma. Therefore, a mixed generalized linear model was used, through GLIMMIX procedure of the statistical package SAS version 9.3 (SAS Institute Inc. 2013) and function of Log link for the analysis of variance according to the design with means repeated in the time. The model considered time as fixed effect, intercept as random effect and repetitions as subject. Components of variance (CV) was the variance-covariance structure of the best fit. The comparison of means at P < 0.05 was performed with the test of fixed range Tukey-Kramer (Kramer 1956). From the results of OD in the exponential stage of the culture, in each repetition, the ln OD was calculated. With these values, maximum growth speeds of strains were determined by linear regression and time of duplication (td) was calculated according to the equation t (d)=ln2/μ (Prescott et al. 2003). Statistics of mean and standard deviation were determined to these data.
Growth of P. pentosaceus in a culture medium with FRUCTOICA as an energy source. A completely randomized design was used with a 2x2 factorial arrangement and three repetitions per treatment. Factors were lactic strains (LB-12 and B-25) and culture media (2). MRS (Oxoid, UK, pH 6.31 ± 0.02) medium was used as control cultivation medium and MRS medium. Glucose was replaced by prebiotic FRUCTOICA (pH 5.74 ± 0.02). It was sterilized by filtration and later added to the sterilization of culture media, at a concentration of 20 g•L-1. As experimental units, tubes with culture media were used according to experimental treatments. They were inoculated with bacterial suspensions at a rate of 1/10 (v/v) to obtain an initial concentration of 106 cfu•mL-1, which was confirmed through a culture in agar MRS plates. Tubes were incubated at 37 ºC for 24 h. After this time, 1 mL was taken from each repetition to determine growth ability (cfu•mL-1) and pH of the remaining culture was measured with a digital pH meter Professional Meter- PP-25 (precision ± 0.01 units). Concentration of viable microorganisms (cfu•mL-1) was determined by visual counting of colonies in agar MRS. For this, samples were dissolved consecutively in a saline solution (0.85%, w/v) and it was cultivated in agar MRS plates, which were later incubated at 37 ºC from 24 to 72 h.
Antimicrobial activity. Antimicrobial activity of lactic bacteria (producers) was determined by triplicate, according to the double layer method (Tagg et al. 1976). Pathogenic bacteria Salmonella enterica subsp. enterica serovar Typhimurium ATCC 14028, Shigella sonnei ATCC 11060, Escherichia coli ATCC 4238, Enterococcus faecalis ATCC 19433, Listeria monocytogenes ATCC 15313 and Shigella flexneri (clinical isolate belonging to LEFM/ICB/UFMG) were used as indicators. For the assay, 5 μL of each active culture was deposited on MRS agar plates and incubated at 37 °C for 24 h. After incubation, the producing bacteria were exposed to chloroform for 20-30 min. Residual chloroform was let to evaporate and plates were covered with the pathogenic cultures. These indicator bacteria were also activated with two subcultures in 5 mL of BHI (Brain Heart Infusion, Oxoid, UK) medium, incubated at 37 ° C for 24 h. An amount of 10 μL of pathogenic cultures were added in tubes with 3.5 mL of semi-solid BHI (0.75 % agar, w/v), homogenized and poured into the plates treated with chloroform. After solidification, plates were incubated at 37 °C for 24 h and the presence or absence of inhibition halos was observed. Inhibition halos indicated antimicrobial activity of strains and their reading was performed with a digital vernier caliper (Mitutoyo Corporation, Kawasaki, Japan). The diameter of bacteria culture was subtracted from the value of halos and differences among means were determined.
Data of microbial growth in a medium with FRUCTOICA and the assay of antimicrobial activity were processed with the statistical package InfoStat version 2012 (Di Rienzo et al. 2012). The multiple comparison test of Duncan (1955) and the t-student were used, when necessary, to determine differences among means at P ˂ 0.05. In the case of microorganism counting, data did not follow the normal distribution, so they were transformed according to logX.
Susceptibility to antimicrobials. Susceptibility was determined by the agar diffusion method with the use of antimicrobial discs, according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI 2012). The antimicrobials Ampicillin (10 μg), Chloramphenicol (30 μg), Clindamycin (2 μg), Erythromycin (15 μg), Gentamicin (10 μg), Kanamycin (30 μg), Nalidixic acid (30 μg), Streptomycin (10 μg), Tetracycline (30 μg), Trimethoprim-sulfamethoxazole (1.25/23.75 μg) and Vancomycin (30 μg), were evaluated, which were selected according to the recommendations of the European Food Safety Authority (FEEDAP 2012). Active strains were cultivated in MRS medium incubated at 37 °C from 18 to 24 h. The test was carried out by duplicate and other lactic bacteria of Lactococcus and Lactobacillus genus, previously isolated and characterized, were used as controls (Pérez-Sánchez et al. 2011 and García-Hernández et al. 2016). The presence or absence of inhibition halos was considered as sensitivity or resistance, respectively.
Results and Discussion
Growth dynamics of P. pentosaceus (LB-12 and LB-25) in MRS medium at 37 ºC are shown in figure 1. Both strains had a similar performance and apparently, their adaptation phases are inferior to 0.5 h. From this time until approximately 4.0 h, the exponential stage was observed. Later, a deceleration phase and after 6 h of cultivation, they reached the stationary stage. table 1 shows growth characteristics of bacteria. The maximum growth speeds of strains were 0.5 h -1 and duplication times of 1.3 h, which is in correspondence with reports for lactic acid bacteria (Schepers et al. 2002).
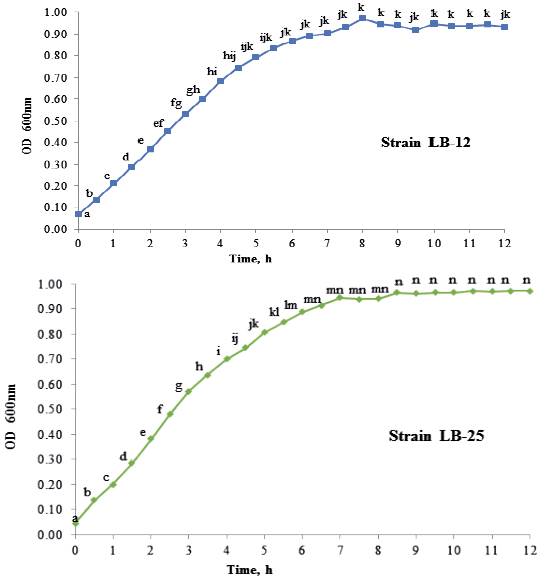
a,b,c,d,e,f,g,h,i,j,k,l,m,n Means with different letters in each time differ at P < 0.05 (Kramer 1956)
Figure 1 Growth dynamics of P. pentosaceus, strains LB-12 (A) and LB-25 (B), in MRS medium at 37 ºC.
Table 1 Growth characteristics of strains LB-12 and LB-25 in MRS medium at 37 ºC.
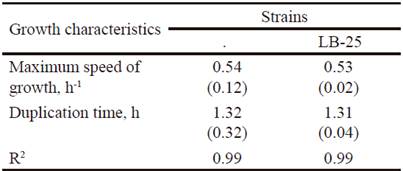
( ) Standard deviation values
Table 2 shows microbial concentration and pH values at 24 h of fermentation at 37 °C in MRS media and in MRS with FRUCTOICA (MRS-F) as energy source. There was interaction for these two indicators and it was observed that the two strains grew in the culture medium where glucose was replaced by fructans of A. fourcroydes up to concentrations of 107 and 108 cfu•mL-1, with values lower than the control. The lowest concentration was obtained for strain LB-25 in the medium with fructans. Apparently, both strains are able to synthesize the enzymatic complex fructosyltransferase, which allows it to use the energy source, although they showed different performances in the presence of fructans. These variations could be associated to physiological characteristics of each strain under study despite coming from the same microbial species. In addition, it must be taken into account that these fructans have complex structures (García-Curbelo et al. 2015b) that are more difficult to degrade, so perhaps Pediococci would need better fermentation conditions to achieve the results obtained with the control medium, especially strain LB-25. These aspects should be confirmed in further studies.
Table 2 shows that both strains of Pediococcus decreased the pH of the MRS medium in more than two units, without differences between strains. However, in the medium with fructans, the decrease was lower, despite the pH value of this medium before inoculation (5.74 ± 0.02) was lower than control medium (6.31 ± 0.02). This performance depended on the strain, because LB-25 had a lower variation of pH, which should be related to its growth ability in this medium with fructans and the concentration of fermentation metabolites. In addition, it is known that the metabolic profile of lactic acid bacteria may vary when different sources of nutrients or culture conditions are used (Papagianni 2012).
Table 2 Microbial concentration and pH values at 24 h of fermentation at 37 ºC in MRS medium and MRS with FRUCTOICA (MRS-F)
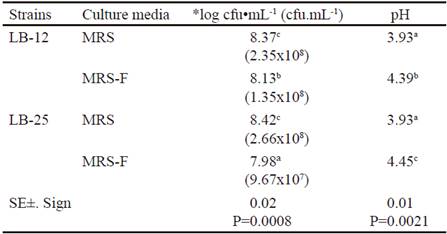
a,b,c,d Means with different letters in each column differ at P < 0.05 (Duncan 1955) *Data were transformed according to log10 (X) because they do not follow a normal distribution
( ) means of the colony forming units per milliliters (cfu•mL-1)
Nevertheless, it should be noted that the fact that both Pediococcus grow and produce metabolites of interest, when fructans of A. fourcroydes are used, it would be possible to include them in symbiotic formulations. In turn, symbiotics when added to the diet can exert synergistic effects by improving the survival, implantation and persistence of probiotic candidates in the gastrointestinal tract (Anadón et al. 2016) and, consequently, decrease the populations of pathogens.
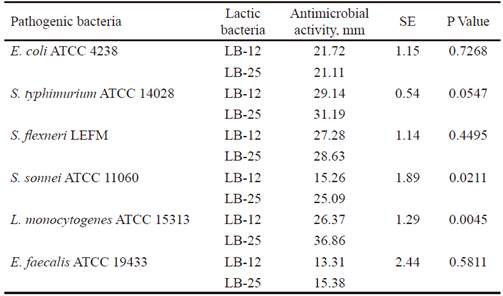
Inhibitory activity of the candidate strains to probiotics have an important function in the competition with other microorganisms in the gastrointestinal tract, for their protection against pathogens (Abbasiliasi et al., 2017). table 3 shows that P. pentosaceus (LB-12 and LB-25) inhibited the growth of pathogenic bacteria populations used as indicators (E. coli, S. enterica subsp. enterica serovar Typhimurium, S. flexneri, E. faecalis, S. sonnei and L. monocytogenes). The activity against these last two pathogens differed among strains and was higher with LB-25. In all cases, except for LB-12 against E. faecalis, the inhibition halos were superior to 15 mm. Results indicate that inhibition or suppression of pathogenic microorganisms could be one of the action mechanisms of the candidates under study. This, in turn, could be related to their ability to produce lactic acid (13.35 and 11.83 g•L-1, respectively), previously determined by García-Hernández et al. (2016), which consequently would contribute to the improvement of intestinal health of the host. In addition, it is known that several strains of this species produce antimicrobial substances that could inhibit the growth of pathogenic bacteria (Martino et al. 2013 and Yasutake et al. 2016). This subject will be the subject of further research with strains LB-12 and LB-25.
Candidate bacteria to probiotic were sensitive to penicillin G, florfenicol, chloramphenicol, amoxicillin clavulanic acid and doxycycline. Likewise, they were resistant to Trimethoprine-Sulfamethoxazole, Cephalexin, Tetracycline, Vancomycin and the evaluated antibiotics of quinolone group (Nalidixic acid, Oxalinic acid, Enrofloxacin and Flumequine) and aminoglycosides (Streptomycin, Kanamycin and Gentamicin). This study is considered as very important according to Ocaña et al. (2006) and it is recommended to expand it in order to find resistance genes (FAO and WHO 2002). In addition, due to the importance of guaranteeing the safety of the use of Pediococcus strains in probiotic formulations, researches should be carried out to demonstrate their status of security, absence of resistance to acquired antibiotics and virulence factors, as well as cell viability in the products ( Porto et al. 2017). These aspects will also be taken into account in subsequent researches.
It is concluded that P. pentosaceus (LB-12 and LB-25) strains show, in vitro, potential as candidates for probiotic for their use in probiotic and symbiotic formulations intended for animal production.











 text in
text in 
 Curriculum ScienTI
Curriculum ScienTI