Occasionally, crop productivity is affected by several stressful environmental factors. It is known that water stress, caused by drought, limits growth and crop productivity, especially in arid and semi-arid areas (Yang et al.2008).
Considering any abiotic stress, there is significant decrease in photosynthesis and consequently, reduction in the amount of metabolites and energy. It is very important for plants to use this small amount of resources to maximize their growth and reproductive potential (Timmusk et al. 2014). The rhizobacteria can contribute to plants tolerating the effects of drought better. Among rhizobacteria, rhizobia occupy an important place in the induction of stress tolerance in plants (Stiens et al.2006).
The filamentous fungus Trichoderma spp., is an effective antagonist against phytopathogens (Howell 1998). Other studies had showed the induction of defense mechanisms in plants by this fungus, as well as its plant growth promoting activity (Saber et al. 2009 and Shaban and El-Bramawy 2011). There is a history of the positive effect of T. harzianum on wheat, when combined with rhizobia (Bécquer et al.2015) and in triticale under agricultural drought conditions (Bécquer et al.2016b).
The objective of this study was to evaluate agroproductive variables of Zea mays L., to which inocula of Bradyrhizobium sp. and Trichoderma harzianum were applied in different combinations and in a simple way and thus select the best options for its practical application.
Materials and Methods
Location of the experiment. The experiment was carried out from February to May 2016, in an experimental plot belonging to the Estación Experimental de Pastos y Forrajes from Sancti Spíritus, located at 21 º53'00 "north latitude and 79º21'25" west longitude ,at 40 m o.s.l.
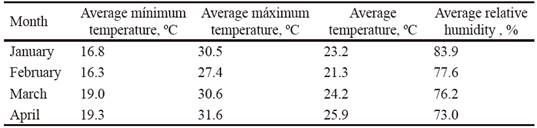
Climate and soil. The temperature, precipitations and relative humidity data were taken from the Sancti Spíritus Meteorological Station (CMP 2016). The precipitations in the study area had an irregular performance (figure 1). During January and April they were higher than historical values, while February, March and May had lower values. The experimental period was characterized by the predominance of high temperatures, especially in April. There was high relative humidity in January, but relatively low in the remaining months, especially in February, March and April, which coincided with the sowing and development of the crop (table 1).
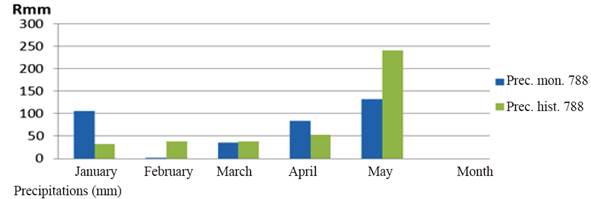
Figure 1 Distributionof precipitation by months and historical rainfall from January / 2016 to May / 2016
The soil of the experimental area corresponded to the soft brown carbonated type, of brown to brownish slightly dark clay, with a slight reaction to HCl. It has some gravels on the horizon A1, good superficial and internal drainage, moderately erodible (Hernández et al.2015). The content of macronutrients was low in phosphorus and potassium (2.63 mg/100 g of P2 O5 and 6.0 mg/100 g of K2 O), as well as in organic matter (1.51 %). The pH was slightly acid (5.9).
Plant material. Corn (Zea mays L.), TGH variety, from the Provincial Seed Enterprise of Sancti Spíritus was evaluated. In the province, this cereal has a history of high yields when inoculated with rhizobia (Bécquer et al.2011) and constitutes, jointly with meadow grasses, one of the possible alternatives for animal feeding in Cuba.
Soil preparation, sowing, irrigation and control of pests and diseases. Conventional culture labors were carried out: plow, harrow, cross, cross again, harrow and furrow. The sowing of the experiment was carried out on the second third of January and harvested in the third ten of April. The sowing dose was of 12 kg/ha with drilling machine. The sowing frame was of 70 cm between furrows. Each plot measured 2.10 m x 4 m, with three furrows per plot. The samples were taken in the middle furrow (5 samples/plot/replication) and the remaining furrows were considered as border effect.
In the experiment, the irrigation was applied four times, twice during February, one in March, and another in April, at a rate of 100 m3/ha in each irrigation, in such a way that only favored the survival of the rhizospheric microorganisms that were introduced. This frequency of irrigation only constitutes 30 % of the average number of irrigation, as well as 22.8 % of the total volume (1775.4 m3/ha) used by Montero et al. (2012), in corn. At 95 days of sowing, the harvest was manually conducted.
Four applications of Bacillus thuringiensis biovar 26 were performed. from 15 days of sowing, every 7 days, at a rate of 6 L/ha. The biopreparation was applied with a sprinkler whose spout was directed to all parts of the plant, with emphasis on the leaf part, in a dilution with water of 1:15 until reaching 16 L of total volume with an initial titre of 109 spores/mL (Central Pesticide Registry 2008).
Microorganisms. The strain Ho13 belonging to Bradyrhizobium sp. genus (Bécquer et al. 2016a) was applied which is microsimbion of Centrosema virginianum, legume from an arid livestock ecosystem of Holguín, Cuba. The product TRICHOSAVE 34 (LABIOFAM S.A.), composed of a shell and rice head substrate inoculated with sporulated mycelia of Trichoderma harzianum A-34, was also used.
Preparation of Bradyrhizobium inocula. The strains grew in solid yeast-mannitol medium (Vincent 1970) and they were re-suspended in liquid medium until reaching cell concentration of 106-108 UFC/mL. For the inoculation of plants, the inoculum was diluted in a 1:10 ratio in 0.9 % saline solution.
Preparation of Trichoderma inocula. The aforementioned product, by technical recommendation of the manufacturer, was added to water, at a rate of 35 g/L. It was filtered with gauze before inoculating the plants (1-2x109 conidia/g) and subsequently. The conidial suspensions were inoculated to the plants according to the treatment used, in a simple way, or in combination with the bacterial inocula. The final title of the suspension (106-108 conidia/mL) was used according to what was proposed by Wolffhechel and Funck-Jensen (1992).
Inoculation of plants with Bradyrhizobium. The inoculation was carried out when the seed germinates (6 d), for which a graduated burette was used, whose content was poured on the newly germinated plants, so that when regulating the spout each plant received approximately 8-10 mL of the liquid inoculum. The re-inoculation of the treatments was performed at 15 d of sowing.
Inoculation of plants with Trichoderma. The inoculation was carried out when germinating the seed (6 d), with a dose equivalent to 250 L/ha of that solution. The same procedure was used as for the Bradyrhizobium inoculum. The re-inoculation of the treatments was conducted at 15 d of sowing.
Fractional inoculation. It was carried out according to the treatment, 15 d after the initial inoculum of the microorganism was applied.
Determination of the agricultural drought state. It was performed using the aridity index or agricultural drought index (EI), according to Solano and Vázquez (1999). This was used to check if the experiment was carried out under water stress conditions:
were:
E o AET |
- Estimated actual evapotranspiration, dependent on soil humidity state. |
Eo o PET |
- Estimated potential evapotranspiration, dependent on atmospheric conditions. |
When AET = PET, the water supply of the soil is adequate. When AET <PET there is insufficient water. It was observed that the highest losses of soil humidity occurred in February and April (table 2), moment when the harvest was carried out. The state of agricultural drought, in January, March and April with an EI from regular to insufficient. In February, it reached the category of insufficient to critical (table 2). It is considered, therefore, that the crop was subjected to high water stress (CMP 2016).
Table 2 Humidity content in the soil and estimated values of PET and AET (2016)
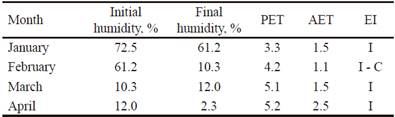
I: Insufficient I-C: Insufficient to Critical R: Regular
Treatments, experimental design and statistical analysis. Table 3 shows the treatments used in the experiment.
A randomized block experimental design was used, with seven treatments and three replications. ANOVA analysis was performed. The differences between means were determined by LSD of Fisher (Fisher 1935).
The values with digit count were transformed by √x and the percentage data by 2arcosen√P (Ruesga et al. 2005). The statistical program StatGraphics Centurion (Stat Point Technologies 2010) was used.
Variables evaluated. They were taken at the time of harvest by replication and treatment. It was determined dry weight of the aerial part (foliage without corncob, DWAP, g / m2); stem length (SL, m); number of leaves / plant (NL); grain yield (GY, kg / ha, extrapolated); corncob length (CL, cm); dry weight of the corncob (DWC, g); weight of 1000 grains (P1000G., g) and inoculation efficiency index (IEI,%) according to Santillana et al. (2012):
Results and Discussion
Stem length (m). Table 4 shows that the Ho13 + Trichoderma (2.03 m) treatment showed higher values (P <0.05) compared to the absolute control (1.84 m) and fertilized control (1.83 m), while shared common superscripts with the rest of treatments. . According to Mia and Shamsuddin (2010), the synthesis of auxins by rhizobia has been widely showed, so it is not ruled out that in the Ho13 strain there was strong activity of these enzymes. It is possible that the Bradyrhizobium strain, which was applied in different variants, positively influenced on this variable by the emission of these plant growth stimulating substances, although Pecina-Quintero et al. (2005) considered that the response of the plant to inoculation depends on several factors, among which is the plant genotype. The Trichoderma performance is not obvious, since diverse authors (Tsavkelova et al. 2006) showed that molecules similar to cytokinin (substance that stimulates cell division in plants), possibly, kinetin, can be produced by T. viride. According to Stewart and Hill (2014), T. harzianum induces growth promotion in many commercial plant species.
Table 4 Results obtained in stem length (SL), number of leaves NL/plant), dry weight of the aerial part (DWAP) and inoculation efficiency index based on the DWAP (IEIDWAP)
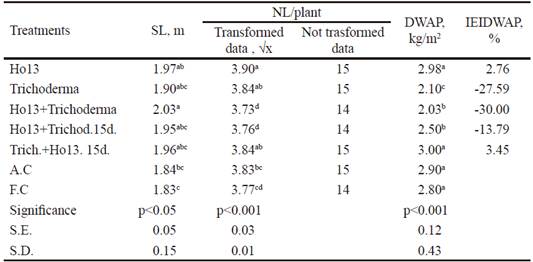
abcdDifferent letters per columndiffera at P<0.001 (Duncan 1955)
Number of leaves/plant. The treatment inoculated with Ho13 (3.90) strain showed common superscripts in Trichoderma and Trich. + Ho13.15d. (3.84, respectively) in the number of leaves/ plant, but it was higher (P <0.001) than the rest of treatments (table 4). Cho et al. (2008) and Yang et al. (2008) considered that the response of plants to water stress includes an increase in abscisic acid (ABA), which causes the closure of stoma to minimize water loss. Due to the crop was subjected to this type of stress, it can be deduced that Bradyrhizobium, being in the rhizobacteria group with induction properties of systemic tolerance to environmental stress, produced cytokinins that counteracted the negative effect of abscisic acid in the leaves. It is known that the colonization by Trichoderma also frequently improves the growth of roots and influences on the crop productivity, as well as the resistance to abiotic stress and the intake and use of soil nutrients (Saba et al.2012).
Dry weight aerial part (kg/m2). For this variable (table 4), inoculated treatments were inferior or shared common superscripts with absolute control (2.90 kg/m2) and fertilized control (2.80 kg/m2). The absolute control and the fertilized control shared common superscripts with two of the inoculated treatments and were superior to the rest, which suggests that there was no proven effect of the treatments inoculated in this variable. In addition, there was deficient absorption of mineral nitrogen by the plant in the fertilized control, perhaps caused by the lack of humidity necessary for this process, derived from the drought stress to which the experimental crop was submitted. The humidity values in the experimental period should have implied an increase in the evapotranspiration of plants, which increases the water demand and consequently, generates higher water stress in the crops (CMP 2016). Nisha et al. (2007) commented that arid and semi-arid regions, as well as deserts, have physical properties in the soil that are very poor, as well as low fertility and water deficiency.
Inoculation efficiency index based on aerial dry weight (%). Although in the variable that was previously discussed, the values of treatments Trich. + Ho1315d. and Ho13 statistically equaled those of absolute control, the inoculation efficiency index (table 4) showed moderate increase of these values (2.76 and 3.45 %, respectively) in comparison with absolute control. However, these percentages are lower than those obtained by Antoun and Prévost (2000) with commercial strains of Bradyrhizobium japonicum, with increases in the ADW of the plant from 6.7 % to 8.7. Contradictorily, Cassán et al. (2009) reported about a strain of Bradyrhizobium japonicum that stimulated germination and incipient development of the aerial part of corn. This strain produced abundant indoleacetic acid, zeatin and gibberellic acid. It is inferred that the Bradyrhizobium strain used in this experiment did not exert the necessary effect on the aerial part of the plant, perhaps due to the insufficient production of hormones, since a negative increase of the treatments was observed with respect to absolute control.
Corncob length (cm). In this variable (table 5), the treatments inoculated with Ho13 + Trich.15d. (20.38 cm) and Trich. + Ho13 15d. (20.67 cm) showed superscripts higher (P <0.001) to absolute control (18.15 cm) and to the fertilized control (18.14 cm), as well as to the rest, except Ho13 (19.65 cm), with which shared common superscripts.
It is notable that these inoculated treatments were superior to the fertilized control, which was also achieved in stem length (table 4) with the Ho13 + Trichoderma treatment. Diverse can be the mechanisms by which the rhizobia can positively affect the crops. Ahmad et al. (2008) found that 80 % of dinitrogen fixing bacteria produce indoleacetic acid. This growth substance leads to the increase of total phenols, calcium content and the activity of the polyphenol oxidase enzyme, which protects the plant against pathogens and improves its growth by eliminating reactive oxygen species that are formed in the plant from a water stress (Chowdhury 2003).
It is obvious to note that the application of Trichoderma at the time of sowing and at 15 d led to statistically higher values, so it is inferred that this fungus exerted a stimulating effect on this variable. According to Gravel et al. (2006), T. atroviride can produce and degrade indoleacetic acid (IAA), the most common auxin phytohormone in plants, in addition to possessing 1-aminociclopropane-1-carboxylic acid diaminase activity, which can control the ethylene production of in the plant under water stress.
Dry weight of the corncob (g). For this variable, the treatments inoculated with Trichoderma (120.30 g), Ho13 + Trichoderma (118.25 g) and Trich. + Ho1315d. (119.90 g) were statistically higher (P <0.001) to absolute control (92.10 g) and to the rest of the treatments (table 5).
Table 5 Results obtained in corncob length, dry weight of the corncob, grain yield, inoculation efficiency index based on GY and weight of 1000 grains
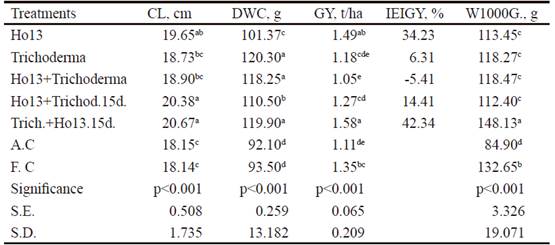
abcdDifferent letters per columndiffera at P<0.001 (Duncan 1955)
Bécquer et al. (2011) obtained promising results in this variable, when inoculating corn in a field experiment with Bradyrhizobium sp., strains isolated in stressful livestock ecosystems from Sancti Spíritus, Cuba. The simple application of Trichoderma favored this variable, which may be linked to the fact that the production of organic acids by Trichoderma favors the solubilization of phosphates, micronutrients and mineral cations, such as iron, manganese and magnesium, which is why the plant absorbs them better (Harman et al. (2004).
The combination of Bradyrhizobium sp. strain with Trichoderma positively influenced on the variable that is described. If the water stress to which the experiment was subjected is taken into account, it is completely logical to infer that the Trichoderma strain, applied, especially, in synergy with Bradyrhizobium, exerted a positive effect on the plants based on these biochemical bases.
Grain yield (t/ha). This variable (table 5) showed that the treatment inoculated with Ho13 (1.49 t/ha) and the treatment inoculated with the combination Trich. + Ho13. 15d. (1.58 t/ha) were higher (P<0.001) to the rest, in which the fertilized control is included (1.35 t/ha).
In the treatments where, at the same time, the Bradyrhizobium and Trichoderma strain, as well as Bradyrhizobium, were applied, in a simple way, they showed higher results, even with respect to the fertilized control. Many rhizobacteria contain the enzyme 1-aminociclopropano-1-carboxylic acid (CCA) diaminase, which cleaves the CCA precursor, ethylene, into α-ketobutyrate and ammonium. Therefore, it reduces the levels of ethylene in plants subjected to stress (van Loon 2007), which allows the root system can develop without the inhibition of this compound. This promotes higher absorption of nutrients by the plant.
The positive results of the treatment with Trichoderma at the time of sowing and Bradyrhizobium sp. at 15 d, it could be due to the cellulolytic effect of Trichoderma on the roots, which allowed effective infection by the bacteria.
Inoculation efficiency index based on GY (IEIGY, %). In this variable (table 5) the results in the grain yield were corroborated, since the treatments Ho13 (34.23 %) and Trich. + Ho13.15d. (42.34 %) showed higher increases than the rest. Cardoso et al. (2007), reported increases of 11 % in corn grain yield, when inoculated with rhizobia. According to Mia and Shamsuddin (2010), inoculation with rhizobia produced a 16 % increase in grain yield in the different varieties of rice. The combination Ho13 + Trichoderma showed negative results, which indicates no influence of this treatment on the grain yield. The fact that the microbial combination described above did not positively influence on this variable, despite its higher results in stem length and dry weight of the corncob, could indicate that the metabolites derived from these microorganisms in this combination did not intervene in the filling of grains. Salinas and Soriano (2014) observed that the coinoculation of T.viride and Bradyrhizobium yuanmingense in Capsicum annuum did not result in higher values for length of stem, leaf, number of lateral roots and dry weight of the aerial part, and it result for root length and dry weight of the radicular part. This could show some inconsistency of the effect of microbial combinations on plants.
Weight of 1000 grains (P1000G). It was state that the treatment inoculated with Trich. + Ho13.15d. (148.13 g) was statistically higher to the rest (table 5). In this variable it was also observed that this treatment showed higher results, so that grain quality was also influenced by the mechanisms described by Kumari et al. (2009) about the absorption of nutrients by the plant, due to the stimulatory action on the root by the PGPRs. Mehboob (2010), when applying Mesorhizobium ciceri strains in corn under greenhouse conditions, obtained statistically higher values than the non-inoculated control. It is possible that the cellulolytic action of Trichoderma on the walls of the root system, or its biocontrol effect, allowed the survival of Bradyrhizobium, as well as its stimulating effect on the plant.
It is concluded that, in general, the productivity of the crop was increased when inoculated with the simple application of Bradyrhizobium sp. (Ho13), as in combination with Trichoderma harzianum. The variables with the best response to the treatments inoculated were: dry weight of the corncob, corncob length and grain yield. The fertilized treatment was inferior to the treatments inoculated in the variables dry weight of the corncob and weight of 1000 grains. The treatments with higher results in the studied variables were Ho13 and Trich. + Ho13. 15 d., which are recommended to apply in agricultural practice under environmental conditions similar to those of this experiment.











 text in
text in