Beneficial effects of supplementation of insulin as prebiotic of fructooligosaccharides on monogastric animal nutrition have been recently discussed Kozlowska et al. 2016). As it is known, fructose polymers, like insulin, are probiotics that resist enzymatic digestion in the prececal area from the feeding canal, and arrive almost intact to the colon, for later disappearing by intestinal fermentation. All these fructose polysaccharides are bifidogenic and stimulate growth of beneficial bacterial species (Kelly 2008).
Among fructose polysaccharides, there are oligofructans obtained from Agave tequilana, which is sown in great plantations from the Mexican state of Jalisco. There are many studies on fructose polymer performance, like oligofructans, on nutrition of recently weaned piglets, due to its abilities to change intestinal microbial population and due to its consequences on animal health (Jensen et al. 2010 and Zhao et al. 2012). However, there are less studies on characteristics of zootechnics of fattening pigs and its possible effect on carcass traits and meat quality (Verdonk et al. 2005).
The objective of this experiment was to determine carcass and performance traits, as well as indexes of technological quality of meat in pigs fed conventional diets of cereals and grains, to which a variable level of agave oligofructans (Agave tequilana) is added.
Materials and Methods
A total of 84 Yorkshire x Landrace x Duroc pigs, female and castrated male in the same proportion, were used. These animals were housed in groups of two, to be randomly assigned to three treatments that consisted in the addition of 0, 2.5 and 5.0 g of agave oligofructans (Agave tequilana) per kg of diet. These oligofructans were obtained from an industrial plant in Jalisco, and came from plantations of jalisciense agave. Pens, of 2 x 2 m of surface, had concrete floor and were located in an open stable. Each of these pens contained feeding and water troughs.
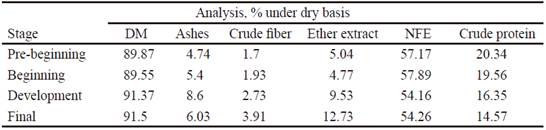
Animals were fed ad libitum for four stages: pre-beginning, beginning, development and final, with typical diets of cereals and grains from 28 d of age, with an initial weight of around 7 ± 0.50 kg. These diets were formulated to fulfill nutritional requirements recommended by NRC (1998). The content of nutrients of diets for the different stages that animals went through were determined in representative samples of food, according known procedures (AOAC 2005). Table 1 shows characteristics of diets.
In order to calculate daily food intake, per week, once the leftovers are weighed, the average per pen is determined. For calculating daily weight gain, pigs were weighed at the beginning and at the end of the test, between four and 23 weeks. Once the performance test was over, the content of dorsal fat was determined in vivo, at the level of the eleventh rib, with a proper ultrasound equipment.
Animals were slaughtered after a fasting of 10 hours, in order to determine carcass yield and main cuts. The slaughtering took place after pigs were stunned using electric clamps. After pigs were slaughtered and bled, pH, color and losses per leaking were measured, in samples of loin at the level of the eleventh rib. The pH was determined 24 hours post mortem, with an ad hoc electrode, connected to a potentiometer. Color was measured with a reflectometer sensor, estimating luminosity “L”, and “a”, tendency to red or green color, and “b”, tendency to yellow or blue color. To measure losses by leaking, a sample of 100 g from the loin was taken, it was weighed at the beginning, at 24 h, at 120 h, and after being placed in polyethylene bags suspended by a thread. This way, it was avoided the sample to touch the walls or covers of these bags. During the measured time, samples were refrigerated at 0°C in a cold chamber.
Besides the measures of carcass traits and meat quality, at the moment of pig slaughtering, cecal content was collected in five animals per treatment, randomly selected, in order to determine the content of short chain fatty acids (SCFA) in the digesta of this digestive organ. SCFA were identified and quantified in samples conveniently homogenized and prepared in the lab through liquid-gas chromatography in capillary column, with the use of 2-ethyl butyric acid as internal standard (Richardson et al.1989).
Experimental data were processed by a completely randomized model (Steel et al.1997). Treatments consisted on the use of different concentrations of oligofructans in the food. The following model provides details:
where:
y |
- variable to be measured |
µ |
- general mean |
V i |
- the i-th level of oligofructan addition |
R j |
- the j-th effect of repetition |
Є |
- standard error |
In the cases measures were significantly P < 0.05) different among them, they were separated by Fisher test, with 95 % of reliability margin. Data were processed with a proper statistical package (Statgraphic 1998).
Results
Table 2 shows data belonging to productive performance of animals.
Initial weight of animals did not differ (P > 0.05) among treatments. Pigs treated with 2.5 g of oligofructans/kg of diet, consumed less food (P < 0.001) than in the treatment without this additive. Pigs with 5.0 g of oligofructans/kg of diet showed an intermediate response. This was not reflected on final weight, total gain or mean daily gain (P>0.05), although it was noted that all these measures were slightly inferior to those of the treatment without oligofructans. This way, it was evident that food conversion tended (P=0.09) to be better in individuals treated with 2.5 or 5.0 g of this prebiotic per kg of food.
Table 2 Performance traits in pigs fed agave (Agave tequilana) oligofructans
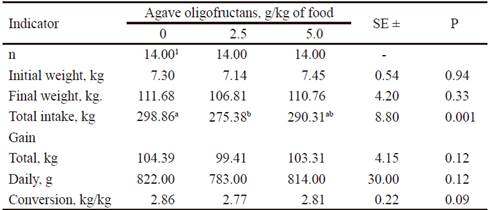
1 Each replication is the mean of two animals
ab Means without common letter in the same row differ significantly (P<0.05) among them
Table 3 shows data referring to carcass traits. Although without significant effect (P>0.05), lean meat yield seemed to be better in both treatments containing oligofructans. With 2.5 g of oligofructans/ kg of diet, the lowest values of dorsal fat thickness were obtained and with 5.0 g/kg, there was superior muscle depth. However, the statistical analysis indicated no significant effect (P<0.05) in these two last measures.
Table 3 Carcass traits in pigs fed agave (Agave tequilana) oligofructans
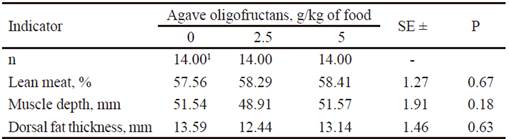
1 Each replication is the mean of two animals
Indexes of technological quality of meat appears in table 4. It was observed that losses by leaking was significantly (P=0.001) low in the groups with the addition of 2.5 and 5.0 g of oligofructans/kg of food, compared to the treatment without prebiotic. Regarding pH measured 24 h post mortem in the meat of control pigs, it was 5.53, which was inferior (P=0.001) to that found in the treatment with more prebiotic (5.86). With the use of 2.5 g of oligofructans in the diet, an intermediate value was found (5.62). Regarding subjective color, it reached a value of 3 with 5.0 g/kg of oligofructans, while there was no effect on marbling of the evaluated samples.
Table 4 Indexes of technological quality of carcass in pigs fed agave (Agave tequilana) oligofructans
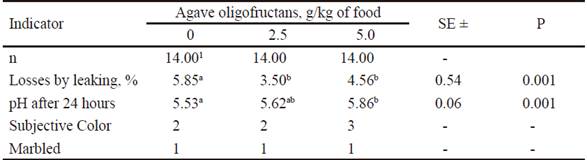
1 Each replication is the mean of two animals
ab Means without common letter in the same row differ significantly (P<0.05) among them
Table 5 shows data related to measurements of luminosity and color (a and b, tendency to red and yellow, in that order). The authors found values of luminosity significantly higher (P=0.04) in meat samples of control pigs compared to those that consumed the prebiotic, without difference between both treatments with oligofructans. Regarding chromaticity, the tendency to red (a) and to yellow (b), showed no influence of treatment (P> 0.05).
Table 6 shows the status of cecal SCFA in pigs. There was significant effect (P<0.01) of treatment in this experiment, when measuring total SCFA concentration in cecal digesta, with high values for samples of animals consuming food with 5.0 g of oligofructans/kg of diet. A lower concentration of the prebiotic in the food did not differ in this index regarding control, without oligofructans. The same happened when concentrations of cecal butyrate, propionate and acetate were measured. In fact, cecal acetate concentration was 1093 µmol/g of fresh digesta, when prebiotic concentration was 5 g/kg of diet, and only 431 µmol/g of fresh digesta in the control treatment. Acetate was the predominant SCFA and, when oligofructans passed from 0 to 2.5 and 5.0 g per kg of diet in these animals, it constituted 55.7, 51.7 and 69.0 % of the total, respectively
Discussion
Results related to performance traits in piglets fed diets with oligofructans have not always been coincident (Verdonk et al. 2005). In addition, such data are scarce, regarding animals in finishing stage (Sobolewska and Grela 2013). Some experiments have revealed evident advantages with the use of this additive in piglets during weaning period Farnworth et al. 1992, Howard et al. 1995 and Olsen and Maribo 1999). However, Shim (2005) obtained increase of liveweight and higher gains in pigs consuming additives in shape of prebiotics. Likewise, Kjos et al. (2010) found increase of weight with the addition of insulin, but these were relatively low, between 4.2 and 6.3 %. Experiments reported by Li and Kim (2013) and by Zhao et al. (2017),with growing pigs and treated with fructose oligomers, showed better performance in animal growth. Data obtained in this research is in accordance with this tendency of finding certain advantage in performance traits when oligofructans are included in the diet of growing and finishing pigs. It is possible that with an inhibition of voluntary intake of food, advantages may be obtained in food conversion of pigs with invariability of daily gain. This decrease of food intake was reported by Urias et al. (2008) in mice handled with this type of prebiotic, probably due to production of sacgenic/incretin peptides, produced in the large intestine of animals that consume oligofructans like those within agave.
Regarding carcass traits, this research could evidence a marked thickness of dorsal fat, like in other experiments with pigs treated with oligofructans (Dezelnne et al.2002). Those results have been attributed to a consequence of the influence of oligofructans, like those used in this experiment, in the lipid metabolism of pigs. It has been proposed that in humans and rats, prebiotics like fructooligosaccharides can modify lipid metabolism, specifically the action of decreasing metabolism of tryacil glycerol through the reduction of novo synthesis of fatty acids by oligofructose action (Delzenne and Kok 1999, Letexier et al. 2003 and Kang et al. 2006).
Sobolewska and Grela (2013) found no effect of weight on the improvement of carcass traits when variable levels of inulin were provided to fattening pigs. This apparent contradiction may be related to the type of fructose polymer included in the diet, as well as its way of supply and dose. Evidently, more research on this subject is needed.
It is evident that changes observed in this experiment on the profile and composition of cecal SDFA are a consequence of using oligofructans on pig rearing. It is very well known that fructose polymers, like oligofructans, are very resistant to enzymatic attack of the host in the stomach and small intestine of animals like pigs, so they reach almost intact o the caecum and colon, where they are attacked by resident microorganisms (Mussatto and Mancilha 2007).
Acetic, propionic and butyric acids are final products of microbial activity that uses hydrolyzed oligofructans (Choct and Kocher 2000 and Sabater et al. 2009). Likewise, it is known that these prebiotics favor the development of bacterial species, like lactobacillus and bifidobacteria, which promote a beneficial path in the balance of native microflora (Banguela and Hernández 2006 and Kelly 2008), in order to favor a better development of gastrointestinal microarchitecture for a higher fermentative activity, among other events. This determines a better health state of animals (Jensen et al. 2010, Zhao et al. 2012 and Samanta et al. 2013).
It is suggested that agave oligofructans may have a positive influence on characteristics of carcass and meat of growing-finishing pigs, with a more marked effect than in performance traits. It is necessary more research to establish the best methods for using oligofructans in rearing pigs.











 text in
text in