Nowadays, swine production undergoes nutritional, social and environmental changes that induce stress responses, which generates greater susceptibility to infection (Pajarillo et al. 2014). Evidently, inadequate management can have immediate negative effects on physiology and performance of the animals (Ponnampalam et al. 2011).
Bacterial infections are the most common in piglets and cause pathological processes that affect the full blood count (Ayala et al. 2008). Generally, antibiotics are used as therapeutic, prophylactic, as well as growth promoters. In many cases, they are used to counteract several pathogens and in some cases, to control outbreaks of infection (Ahmed et al. 2014). The increasing of microbial resistance to antibiotics caused the European Union, since 2006, to prohibit its use as growth promoters in animals (Dadvar et al. 2015). Therefore, taking into account the economic losses caused by diarrheal disorders, it was necessary to look for alternatives to mitigate these physiological disorders in animals (Lye et al. 2012).
Biological media, such as probiotics, are microbial additives obtained from monocultures or mixed cultures, which inhibit the growth of pathogens in the gastrointestinal tract (Colina et al. 2011). Likewise, they play a role in the maintenance of the function of intestinal barrier and local and systemic modulation of the immune system, which contributes to improve the health of the host (Londoño and Parra 2015). Hence, the objective of this study is to evaluate the effect of two microbial preparations on hematological values and blood biochemicals of piglets, at 14 and 42 d of age.
Materials and Methods
The experimental study was carried out in the pig production unit “Gahuijón Alto”, Canton Colta, Ecuador. The unit is located at 1° 53' 12.248" SL longitude 78° 43' 22.454" LW, 3 510 m.o.s.l. (meters over sea level), with annual precipitation between 500 and 1,000 mm. Minimum, maximum and average temperature is 3, 14 and 10 ºC, with annual relative humidity of 80 % and annual evapotranspiration of 69.03.
Experimental treatments and design. A completely randomized design with four repetitions per treatment was used, where each experimental unit was composed by twelve piglets. Evaluated treatments were: T1) control; T2) microbial preparation A and T3) microbial preparation B.
Animals. An amount of 120 Duroc x Landrace/Yorshire crossbreed piglets were used, which descendants of 12 first farrowing sows (Landrace/Yorkshire), with 135.6 ± 2 kg of liveweight (LW) and 235 ± 3 d of age. Four pregnant sows were used per treatment, housed in collective pens of 6 x 6.5 m wide and cement floor, with a density of 1.6 m2 per animal, from gestation to 110 d of gestation. After parturition, piglets were distributed at random to form the experimental groups with 40 animals per treatment (20 females and 20 males). From this moment until weaning, they were settled in maternity area. Piglets after weaning were regrouped (without altering the groups of origin) with 20 piglet, in pens of 6 x 6.5 m in cement floors. The food used was Bioalimentar® (Ambato, Ecuador), which meets the nutritional requirements for pigs, recommended by NRC (2012). Bioalimentar was offered to sows twice a day, at 7:00 a.m. and 4:00 p.m., and the piglets received it ad libitum from 7 d of age up to weaning. From there until 42 d of age, they were fed at the same time as their mothers. Water was also provided ad libitum in nipple troughs.
Animal management system. Maternity was maintained at 28 °C during the first two weeks after parturition. Later, this temperature was reduced in 1.5 °C every week until weaning. Photoperiod was controlled with 12 h of light and 12 h of darkness. The litters of each treatment were located distant from each other (with an intermediate pen on both sides of the corridor) to avoid self-inoculation. Piglets were weaned at 33 d of age. Piglets from each group under study received the proper veterinary attention, according to the Manual de Manejo de Hembras y Primerizas (Coates et al. 2013).
Microbial preparations. The strains used in the preparations were Kluyveromyces fragilis (L-4 UCLV), from the Bank of Microorganisms of the Universidad Central “Marta Abreu” of Las Villas, and four ATCC (American Type Cultures Collection, USA) strains, which were Lactobacillus acidophilus, L. bulgaricus, Streptococcus thermophilus and Saccharomyces cerevisiae. These strains were activated in skim milk at 37 °C for 24 h. To obtain the preparations, the mixture of sugarcane molasses and orange vinasse was used as a substrate and fermented at 37ºC for 24 h, according to the methodology described by Miranda et al.(2017). In preparation A, L. acidophilus, L. bulgaricus and S. thermophiles were used and in B, the previous bacteria plus S. cerevisiae and K. fragilis yeasts (L-4 UCLV) were also used. Chemical composition and microbial concentration of each preparation are shown in table 1.
Table 1 Composition of microbial preparations to be included in the base diet of mother sows and their offspring
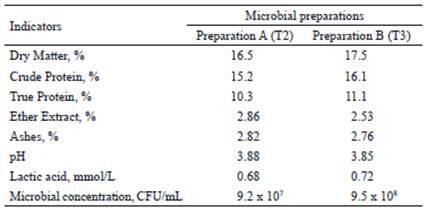
Use of additives in pigs. Microbial preparations were provided to animals of groups T2 and T3 at 7:00 a.m. every three days. The breeding sows received 15 mL of additive, by mixing 0.3 kg of balanced food plus 0.5 L of water, from the day after the confirmation of pregnancy until weaning, according to the assigned treatment. The offspring continued to receive the same additive. The first dose applied to piglets was a single dose before having colostrum. Oral dosage varied according to age: 1 mL in the first week; 1.5 mL in weeks 2 and 3; 2 mL in weeks 4 and 5, and 2.5 mL in the following up to 42 d of age. Control group received normal saline solution in the same amount as the treated groups.
Experimental procedure for taking and analyzing samples. At 14 and 42 d of age of the animals, 12 pigs of each treatment were randomly selected. They were immobilized and extracted 8 mL of blood from the jugular vein. These samples were taken in vacutainer tubes, with and without ethylenediaminetetraacetic acid (EDTA), using a California-type needle. Subsequently, they were transferred to the laboratory within the first three hours for further processing. The evaluation of the blood profile consisted of the determination of hemoglobin (Hb), hematocrit, erythrocyte, mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), leukocytes, basophils, eosinophils, lymphocytes and monocytes, through the methodology described by Kraft (1998) and Corredor (2012). Indicators of blood biochemistry were total proteins, albumin, glucose, triglycerides, total cholesterol, high-density lipoprotein-cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), determined according to Mejía et al.(2012) and Londoño and Parra (2015).
Statistical analysis. The experimental data were processed with the statistical package Statgraphic plus 15.1 for Windows. Analysis of variance was carried out according to a completely randomized design. In the necessary cases, Duncan (1955) comparison test was applied to discriminate differences between means at P <0.05.
Results
Table 2 shows the blood profile of the piglets, evaluated at 14 and 42 d of age. In both ages, differences were found with respect to control for all the evaluated indicators, except lymphocytes at 14 d of age. With the inclusion of microbial preparations, in both measurements, hemoglobin, hematocrit and erythrocytes increased (P <0.05). Hemoglobin was higher in T3, while hematocrit and erythrocytes did not differ between T2 and T3.
At 14 and 42 d, MCV and MCHC were higher (P <0.05) in T1 with respect to T2 and T3, without differences between these groups. This same effect was found for MCH at 42 d of age, not being so at 14 d, when differences were detected between T2 and T3, with a lower value in T3. Leukocytes and basophils also decreased with the inclusion of microbial preparations, without differences between T2 and T3 (table 2).
Table 2 Full blood count (FBC) of piglets at 14 and 42 d of age, when supplemented with two microbial preárations
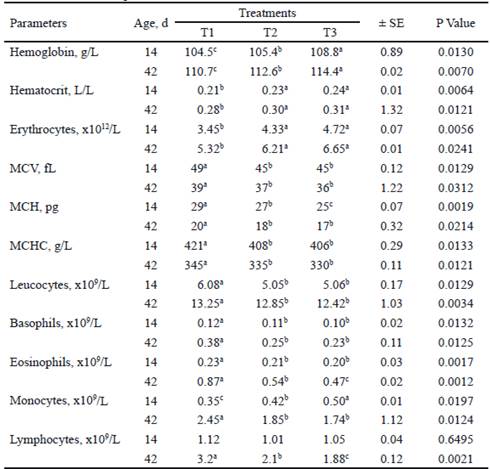
a,b,c Means with different superscripts in the same row differ at P<0.05 (Duncan 1955) T1, Base diet without additive. T2, L. acidophilus + L. bulgaricus and S. thermophilus. T3, L. acidophilus + L. bulgaricus + S. thermophilus + S. cerevisiae and K. fragilis (L-4 UCLV). MCV, mean corpuscular volume. MCH, mean corpuscular hemoglobin. MCHC, mean corpuscular hemoglobin concentration. Blood values used as reference of Kraft (1998) and Corredor (2012)
With respect to monocytes, in the evaluation performed at 14 d, the values were higher (P <0.05) for the animals treated with additives, being superior in T3. However, at 42 d old, this indicator was higher (P <0.05) in the control treatment, without differences between T2 and T3. There were no differences (P <0.05) among treatments for lymphocytes at 14 d. While at 42 d, it was higher (P<0.05) in the animals that did not consume the microbial preparations (T1).
Table 3 shows the performance of blood biochemistry of piglets at 14 and 42 d old. In both ages, differences were found with respect to the control for all the evaluated indicators. Total protein was lower (P <0.05) in the control with respect to the piglets that consumed the microbial preparations. Albumin was also lower (P <0.05) in control animals with 14 d of age, without variations among treatments (T2 and T3). However, the opposite effect was observed for this indicator in the measurement at 42 d ofage.
The glucose values decreased in piglets treated with the microbial preparations. Out of these, the lowest was (P <0.05) in T3, at 42 d of age. Triglyceride, total cholesterol and LDL-C levels were lower in T2 and T3 with respect to animals in control group T1, while HDL-C values were increased in piglets treated with microbial cultures (T2 and T3).
Table 3 Performance of blood biochemistry of pigs at 14 and 42 d of age, supplemented with two microbial preparations

a,b,c Means with different superscripts in the same row differ at P<0.05 (Duncan 1955)
T1, Base diet without additive. T2, L. acidophilus + L. bulgaricus and S. thermophilus. T3, L. acidophilus+ L. bulgaricus + S. thermophilus + S. cerevisiae and K. fragilis (L-4 UCLV). MCV, mean corpuscular volume. MCH, mean corpuscular hemoglobin. MCHC, mean corpuscular hemoglobin concentration. Blood values used as reference of Kraft (1998) and Corredor (2012)
Discussion
Blood profile results of this study (Table 2) could be related to the substrate to ferment the previously mentioned microorganisms and with the microorganisms used and introduced with the diet to the mother sows and their offspring. This could favor the physiological changes in the piglets, possibly by improving the imbalance of the autochthonous microbiota of the gastrointestinal tract, which affected the values of blood indicators. In this sense, the results of this research agree in part with those reported by Ayala et al.(2008), who, when supplementing Bacillus subtilis with sows in the last gestation stage, observed higher (P<0.05) concentration of Hb and Hto in the animals that consumed this bioproduct, which indicates a probiotic effect on health of pigs. Muñoz et al.(2010) reported a slight decrease in physiological changes, as well as in the stabilization of blood values in pigs, by supplementing a mixed culture of lactic acid bacteria, based on the assumption that microbial preparations in piglets could improve passive immunity and reduce blood disorders, such as hemolysis or destruction of red blood cells (Sun et al. 2015). Regarding other blood indicators, values obtained in this study were among the reference limits for this species (Muñoz et al. 2010). However, some blood indicators obtained were favorable for the animals that consumed the microbial preparations, which can be attributed to the fact that probiotics had a favorable influence with a constant contribution of blood-forming elements (Ayala et al. 2008). González et al.(1993) reported similar responses by supplementing microbial additives in pigs with six weeks old.
Regarding blood biochemistry of piglets at 14 and 42 d of age (table 3), it is confirmed that the use of the additives did not cause a negative effect on the animals, since the values of blood biochemistry of piglets were among the values considered normal for pigs by González et al.(1993) and Espinosa et al.(2008). Likewise, uniformity and superior degree of immunity is observed in the treated animals, attributed to the response that microbial preparations with probiotic capacity may cause, associated with eubiosis of intestinal biota that stimulates the response of lymphoid tissue and promotes better health state in piglets (Ahmed et al. 2014 and Dadvar et al. 2015). Similar results were reported by Ponnampalam et al.(2011) and Londoño and Parra (2015), by supplementing microbial additives in pigs. Colina et al.(2011) observed a reduction (P <0.05) in serum levels by supplementing lactic acid bacteria. Muñoz et al.(2010) also reported similar results, which could affirm the beneficial effect of microbial additives on animal health and its favorable repercussion for final consumer of pork (Lye et al. 2012). Pajarillo et al.(2014) and Sun et al.(2015) argued that the beneficial effect of probiotics occurs when they are ingested in adequate amounts and that the intestinal ecosystem of the host is modified. This, in turn, generates a beneficial microbial balance, which means a better animal health.
Another beneficial effect of microbial preparations is to act against hypercholesterolemia, reducing total cholesterol and LDL-C by balancing the natural microbiota of the gastrointestinal tract of the host (Ahmed et al. 2014). This study showed an increase of HDL-C and a reduction of LDL-C at 42 d of age, which may indicate that the animals consuming microbial preparations may have had better functioning of metabolic organs, despite that levels found in all evaluated treatments were within the normal reference range (Colina et al. 2011).
There is evidence of a decrease of postprandial curves of glycemia and insulin after providing S. cerevisiae in diet for goats (Dadvar et al. 2015). It is known for some time that ingestion of non-digestible carbohydrates modifies the kinetics of glucose absorption, reducing postprandial peaks of glycemia and insulinemia (Ros 2003). Changes in intestinal expression of released insulinotropic peptides in response to ingestion of microbial preparations may also have an important function in the lipid effects observed in this study. This could be, in turn, closely related to metabolic diseases and the composition of bacterial populations in the intestine of the host (Lye et al. 2012). In this study, glucose reduction was observed at 14 and 42 d of age in the animals that consumed the microbial preparations, which may indicate that animals had better functioning of metabolic organs, even though the levels found in all treatments were in the normal reference range (Colina et al. 2011 and Mejía et al. 2012).
It is concluded that the addition of the evaluated microbial preparations with probiotic capacity in diets for piglets favorably improves the values of the blood and biochemical profile of the blood and with it, the physiological state and the health of the animals.











 texto en
texto en 


