Xylanases (β 1,4endo-xylanases; EC 3.2.1.8) are enzymes involved in xylan degradation. They hydrolyze internal links β (1→4) among xylose molecules, producing a mixture of xylooligosaccharides with different sizes. The ability of degrading xylan is widely distributed among microorganisms, which have different enzymes with a specific action for the complete degradation of this substrate (Chakdar et al. 2016).
Among the microbial groups, bacteria and fungi are provided with powerful xylanolitic equipment (Chakdar et al. 2016 ). Bacillus genus species are the most used (Banka et al. 2014) because they represent high growth rates that allow shorter fermentation times, have great ability for extracellular secretion of heterologous proteins and are included within the microorganisms considered as GRAS (Generally Regarded as Safe) by ADF (Gupta et al. 2015).
Xylanases are very important at biotechnological level. For example, they have commercial use in paper and textile industry, in the production of detergents, for improving beer and wine fermentation (Chakdar et al. 2016) and, mainly, in food industry, with the bioconversion of cellulosic materials for poultry, with better digestion and nutrient absorption (Schoenlechner et al. 2013 and Hahn-didde and Purdum 2014).
The objective of this research was to evaluate the xilanase production ability of Bacillus subtilis E44 strain under submerged fermentation conditions.
Materials and Methods
Microbial culture. Bacillus subtilis E44 strain was used from the Laboratorio de Microbiología of the Facultad de Ciencias Agropecuarias of the Universidad de Matanzas, preserved at -30 ºC in glycerol.
Culture media. A minimum medium of salts (MS) was used and the mineral solution was composed by (%): NaCl-0.1; KH2PO4-0.3; K2HPO4-0.6; MgSO4-0.12; peptone-0.5 and yeast extract-0.3. The pH was fitted at 7.5 with KOH 1mol L-1.
Broth and agar nutrients were prepared according to indications of the manufacturer (BioCen, Centro de Biopreparados).
Inoculum preparation. From the culture preserved in glycerol at -30 ºC, bacteria were cultivated in a nutrient broth and incubated for 16h at 28 ºC in a shaker at 110rpm. Cellular suspension was cultivated in nutrient agar and preserved at 4 ºC. Using these cells, the pre-inoculum was prepared in 50mL of nutrient broth, incubated at 28 ºC in a shaker at 110rpm for 16 h up to obtaining an optical density (OD 600nm =0.8) equivalent to a concentration of 1x108 cfu/mL.
Qualitative evaluation of xilanase production. Petri dishes were prepared with MS medium plus 1 % of beech xylan (Sigma-Aldrich). Dishes were inoculated by deep culture from the culture of Bacillus subtilis E44, and were incubated at 28 ºC for 24, 48, 72 and 96 h. For revealing hydrolysis halo, dishes were cover by a solution of Congo red at 0.5 %, for 5 min, followed by three washes in a solution of NaCl 1molL -1. Power index (PI) was calculated by the relation between the diameter of hydrolysis zone and the diameter of the colony. Diameters were measured with a vernier caliper (Suertek cap brand. Sensitivity of ± 0.02 mm). The experiment was performed by triplicate.
Submerged fermentation. The experiment was conducted in flasks of 250 mL containing 50 mL of culture media: 1) nutrient broth (NB) and 2) minimum medium of salts plus beech xylan at 0.5%, as carbon source (MS+X). The pH was fitted at 7.5 before sterilizing at 121ºC for 15 min. Flasks were inoculated with a cellular suspension of 1x108 cfu/mL (O.D 600nm= 0.8) of Bacillus subtilis E44, relation 1:10 (v/v) and were incubated in a shaker at 110 rpm for 24 h at 37 ºC.
Determination of growth kinetics. An amount of 5 mL was taken in intervals of two hours for the first 12 hours in both cultures, absorbance (OD) at 600 nm was measured, and later centrifuged at 10 000 rpm for 15 min at 4 ºC. Supernatant was used for evaluating the activity of xylanase and protease enzymes.
Values of growth specific speed (μ) were determined as the slope of polynomials belonging to the exponential phase of dispersion curves, obtained from the growth kinetics of B. subtilis E44 in the evaluated culture media, with the use of fit method of Microsoft Excel program. Using these values, duplication time was calculated (td).
Determination of xylanase enzymes activity. The activity of xylanase enzymes was determined according to the method described by Bailey et al. (1992). A unit of enzymatic activity is defined as the amount of enzyme required to produce 1µmol of xylose per minute.
Determination of protease enzymes activity. The activity of protease enzymes was determined according to the method Anson (1938). A unit of enzymatic activity is defined as the amount of enzyme required to produce 1µmol of tyrosine per minute.
Statistical analysis. Statistical procedures were carried out with the use of InfoStat software (Di Rienzo et al. 2012). For the treatment of power indexes (PI), an analysis of variance was performed according to a completely randomized design and Duncan (1955) test was applied, for P<0.05. Values of tables and graphs belong to the mean of three repetitions.
Results and Discussion
Production of xylanase enzymes by Bacillus subtilis E44 in the presence of beech xylan (1%) as the only carbon source was evident by the presence of clear zones around the colonies after Congo red revealing. This colorant shows strong interactions with polysaccharides joined by β 1.4 links. With the hydrolysis of these compounds, interactions between colorant and polysaccharides are lost and, at the same time, the coloration of the halo belonging to the area in which bacteria secreted xylanases.
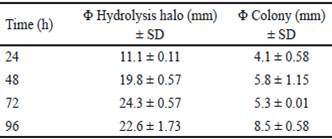
Bacillus subtilis E44 showed a great xylanolitic activity at 24, 48, 72 and 96 h. Diameters of hydrolysis halos showed values between 10 and 25 mm for the evaluated times (table 1). The highest value was obtained at 72 h of incubation with 24.7 mm. these results coincide with those obtained by Akhavan et al. (2011), with the evaluation of 40 strains of Bacillus mojavensis that generated halos with diameters between 18 and 35 mm after coloration with Congo red. On the other hand, Tork (2013) selected 13 xylanases-producing strains, isolated from different samples, which showed halos between 13 and 22 mm of diameter around the colonies.
Table 1 Qualitative evaluation of xylanase enzymes of Bacillus subtilis E44, in beech xylan as the only carbon source
Several authors use this method for a rapid evaluation of extracellular enzyme production by microorganisms (Braga et al. 2014 and Chafla et al. 2016). Even Cayetano-Cruz et al. (2016), evaluated the expression of recombining genes of xylanase (xyn 11 A) in Pichia pastoris through the use of the technique in clones obtained with xylan agar as substratum.
Table 2 shows the analysis of variance of calculated power indexes (PI). The highest PI was obtained at 72 h and coincides with the time in which the highest halo of hydrolysis was obtained. At 96 h, there is a decrease of PI. However, diameter of the colony obtained in this time is superior regarding the value of hydrolysis halo (table 1). This decrease of PI could be associated to the increase of colony diameter due to sporulation of B. subtilis E44, period in which there are no increments of nutrient intake (Milian 2009).
Results indicate that xylanase enzyme production by Bacillus subtilis E44 depends on time, in the qualitative essay in dishes. This indicator has also been used by other authors in the selection of cellulases (Chafla et al. 2016) and feruloyl esterase (Braga et al. 2014) in fungi isolated from different sources. Values obtained in this study surpass those reported by the previously mentioned authors.
Kinetics of Bacillus subtilis E44 growth in the studied media cultures appears in figure 1. In the medium nutrient broth culture (NB), the exponential phase starts very early and it lasts up to 4 hours. From this moment, the phase of deaccelerating of growth begins. However, in the medium MS+X, the exponential phase started at 4 hours and lasted up to 12 h.
Table 2 Power index obtained for Bacillus subtilis E44, in beech xylan as the only carbon source
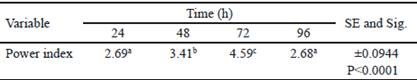
a, b, c Different letters indicate significant differences for P < 0.05
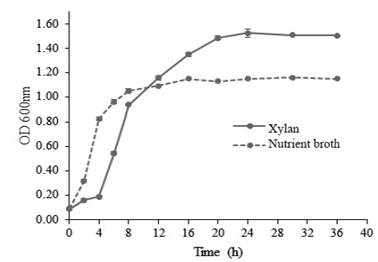
Figure 1 Growth kinetics of Bacillus subtilis E44 in nutrient broth and in a medium of salts plus beech xylan
This performance may be related to differences in the composition of both media. NB contains glucose, which guarantees the availability of a simple carbon source in the medium and it is easy to use by bacteria. In the MS+X, which only carbon source is beech xylan , polysaccharides show complex structures that need, for their degradation, enzymes synthesized by bacteria and later use hydrolysis products as carbon source, which occurs during the logarithmic phase.
The graphic performance of growth is similar to the obtained values of specific growth speed (table 3). Once the exponential phase in the MS+X medium begins, time of duplication is superior to that obtained in the NB medium, which confirms the xyanolitic ability of the strain.
Milián (2009) compared two culture media for the production of B. subtilis E44 with values of specific growth speed of 0.66 h-1 in a traditional culture media and 1.00 h-1 in an optimized culture media with national components.
Similar results were reported by Ho (2015), with the evaluation of different agro-industrial residues as carbon source and beech xylan as control for xylanase production by Bacillus subtilis ATCC 6633 during submerged fermentation. Beech xylan was used as positive control and showed remarkable increases of xylanase activity, while barley peel was used for evaluating growth of the bacteria and activity of these enzymes.
When analyzing growth kinetics (figure 1) and xylanase activity detected during growth (figure 2), it is observed a correspondence between both profiles. Xylanase activity increases since the first hours, the maximum value was detected at the end of exponential phase and maintains high during the stationary phase. Abo-State et al. (2013) reported a direct correlation between microbial growth profile and production of xylanases and cellulases in Bacillus species. Likewise, Ho (2015) informed a correlation between microbial biomass and the expression of xylanases in Bacillus subtilis ATCC 6633.

Figure 2 Xylanase activity of the enzymatic preparation in broth and medium of minimum salts plus beech xylan
One of the major limitations in extracellular enzyme production from species of Bacillus genus is susceptibility of these proteins to degradation by proteolytic enzymes (Westers et al. 2006). The evaluation of proteolytic activity during growth of B. subtilis E44 and its relation with xylanases in the MS+X medium is shown in figure 3.
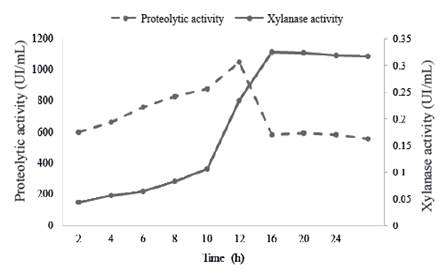
Figure 3 Relation between proteolytic activity (UI/mL) enzymes and xylanase activity of the enzymatic preparation in a MS+X medium
During the exponential phase of growth, there is an exponential increase of protease activity with a sudden increase at the end of the exponential phase and begins to decrease rapidly after 12 h. This decrease of proteases may be related to processes of own deactivation.
These results agree with statements of Sevinc and Demirkan (2011), who reported a strain that produces proteases and the maximum activity occurs at the end of exponential growth phase.
Proteases have important functions in the quality control of denaturalized proteins or with conformational defects after post-translational events (Westers et al. 2008). Peptidases are constitutively expressed and temporarily have different affinities for pre-protein secreting systems (Ling et al. 2007), which constitutes one of the main problems in the production of heterologous proteins in Bacillus subtilis.
These results suggest that protease production is directly related to the exponential growth of culture, characterized by an increase of cellular metabolism and protein synthesis. Cristina et al.(2004) reported similar results in a culture of Bacillus sp. SMIA-2, where protease production reached the maximum value at 9 h and later started to decrease.
Out of the results obtained in the present research, Bacillus subtilis E44 produces xylanase enzymes able to degrade beech xylan and use simple sugar obtained during their growth and development. Difference between times of maximum expression of proteases and xylanases may be used to increase yields of xylanase enzymes during submerged fermentation of Bacillus subtilis E44 in a MS+X culture medium.
It is concluded that Bacillus subtilis E44 produces xylanase enzymes with the use of xylan as carbon source. These enzymes could be used in diets with high fiber content in monogastric animals.











 text in
text in 



