INTRODUCTION
Currently, some studies have being performed to achieve good practices that guarantee a better use of food by animals in order to increase productivity (Toledo et al. 2018). Within these practices, it can be found the administration of safe and stable microorganisms to increase resistance to diseases and improve the nutritional state of animals. Probiotics are postulated as a potential replacement alternative to antibiotics used as subtherapeutics, as growth promoters. Its advantage is that they do not leave residues in the eggs or in the meat of birds and do not generate a risk of antibiotic resistance in the human microbiota (Arteaga et al. 2018). However, they have the disadvantage of high prices and stability over time (Pérez and Sablón 2017).
The choice of an appropriate conservation method is important to keep intact the characteristics of any biological product and suitability (Caicedo and Valle 2017). The existence of different methods of preservation of products with probiotic effect are known, such as lyophilization, microencapsulation and spray drying, among others, in order to maintain its extrinsic characteristics (Zhang et al. 2015 and Rueda et al. 2016). According to Rodríguez et al. (2016) and Molina (2016), one of the elements that affect the survival of microorganisms and conservation over time is temperature and pH. These two indicators are reported as methods of conserving viability of biological products (Montañez and Castro 2006). Hence, the objective of this research was to verify the stability of the SUBTILPROBIO® C-31, C-34 and E-44 zootechnical additives under conditions of refrigeration and room temperature.
MATERIALS AND METHODS
Production of zootechnical additives. Zootechnical additives were obtained at the Centro de Estudios Biotecnológicos de la Facultad de Ciencias Agropecuarias, University of Matanzas. The methodology proposed by Milián et al. (2017b) was used. C-31, C-34 and E-44 strains of Bacillus subtilis, subtilis subspecies, were used, which were isolated and identified by Milián et al. (2014).
To verify the stability of these zootechnical additives at room temperatures (24 ± 3 ° C) and in refrigeration (4 ± 8 ° C), the rack test was carried out, and 15 bottles of both temperatures were taken and the following parameters were measured:
1. Microbiological quality of the additives. To confirm the microbiological quality of zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44 for their use in animals, counting of contaminating microorganisms was carried out according to the regulations in force, described for the Microbiological quality studies of the NC-ISO Human and Animal Consumption Foods according to Bennett and Lancette (2007) (table 1). This sampling was done in both temperatures, and they were taken at the beginning (1st day) and end of the experiment (180 days).
Table 1 Microbiological tests for determining contaminant microorganisms
| Microbiological tests | Reference NC- ISO |
|---|---|
| Recount of total and fecal coliforms | 4832: 2010 |
| Recount of Pseudomonas auruginosa | 4833-1: 2014 |
| Recount of Staphylococcus aureus | 6888-1: 2003 |
| Recount of Bacillus cereus | 4833-1: 2014 |
| Counting of Salmonella in 25 mL | 6579: 2008 |
| Counting of Enterobacterias | 4832: 2010 |
| Recount of viable yeasts per mL | 7954:2002 |
| Recount of fungi | 7954:2002 |
2. Viability of endospores. Samples were taken at 1, 7, 30, 60, 90 and 180 days, and were cultivated on plates with nutrient agar. Incubation was performed at 37 °C for 24 hours under aerobic conditions. Microorganism count was made through the number of colony forming units (CFU). It was determined by visual counting of colonies on plates with nutrient agar.
3. pH dynamics. To determine pH dynamics at both temperatures, samples were taken at 1, 7, 30, 60, 90 and 180 days, and three repetitions were made. The measurement of the pH values was carried out in a digital pH meter (Sartorius Meter PP-25).
Statistical processing. For data analysis, the statistical software INFOSTAT, version 2012 (Di Rienzo et al. 2012) was used. The analyses of variance were performed to verify differences among means, with a significance level of P <0.05. Duncan (1955) test was applied to perform multiple comparisons among means, in the treatments viability of endospores and pH dynamics.
RESULTS AND DISCUSSION
Microbiological quality of zootechnical additives. The results from the microbiological quality study of the zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44, at the two temperatures studied: room (24 ± 3ºC) and refrigeration (4 ± 8ºC) did not show the presence of contaminating microbial agents. The counts of yeasts and fungi were in the permissible ranges (yeast: <103 mL and fungi: <10UFC.mL-1) for the consumption of biological products.
These results can be associated to the ability of Bacillus genus to produce a wide variety of antimicrobial substances, all of a protein nature, which differ in their mode of action and chemical structure, which are discharged outside the cells. This allows them to establish and, in this way, inhibit the presence of pathogenic microorganisms in a biological product, as well as the production of hydrogen peroxide, which is recognized as an inhibitor of the growth of Gram- negative bacteria.
This research corresponds to reports of Flores et al. (2015) about the zootechnical additives, that although it is true that Lactobacillus, yeasts or other group of microorganisms may have a probiotic potential, it is essential to evaluate their microbiological quality, durability over time and components of each product that is used.
In this sense, research carried out by Milián et al. (2017a) and Rodríguez (2017) reported that one of the advantages of Bacillus genus is the ability to produce LFB 112-type bacteriocin and the lipopeptides Surfactin and Mycosubtilin. They inhibit the development of Gram-positive and Gram-negative bacteria, such as E. coli, Salmonella spp., C. perfringens, Streptococcus spp., S. aureus, Pasteurella multocida, and P. aeruginosa. The results are in line with the aforementioned, since the presence of these pathogens was not observed in the zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44, so it can be deduced that the bacteriocins produced by B. subtilis had their effect.
Results homologous to those derivatives in this study, were those reported by Molero et al. (2017), when evaluating the microbiological quality and useful life of fermented probiotic beverages based on whey, obtaining a high level of inocuity, thanks to a low recount of mesophilic aerobes and absence of total and fecal coliforms and S. aureus.
Rondón 2009 and Pérez et al. 2016 performed similar studies to zootechnical additives PROBIOLACTIL® and PROBIOLEV®, where they obtained similar results to this research. The recount of fecal and total coliforms, Staphylococcus aureus, Pseudomonas aeruginosa, and Bacillus cereus, in all cases was negative, fungi (<10 CFU / mL-1), yeasts (<103 / mL) and Salmonella spp. (no presence).
Yeast levels obtained for the three additives are in the permissible range (<103mL), it is inferred that their inhibitory action had an effect on the quality of the additives as an antibacterial agent. Yeasts contain mannan oligosaccharides of (MOS) in their cell walls. These limit the adherence of lectinases to carbohydrates and reduce the colonization of pathogens such as E. coli and Salmonella spp. Many authors refer to the action of yeasts as inhibitors of pathogenic microorganisms (Alcázar et al. 2016 and Rodríguez 2017).
Viability of endospores. Viability of endospores in the zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44, add no significant differences in the endospore counting (16 Log UFC.mL-1) at room temperatures (25 ± 3 ºC) and refrigeration (4 ± 8 ºC). This is explained by the ability of bacterial endospores to survive under extreme conditions. Reports by Espitia et al. (2014) demonstrate this, when they defined that spores of Bacillus contain a large amount of small proteins that resist sudden temperature changes, act against acids, formaldehydes and conservation techniques among others.
Caicedo and Chacón (2017) refer that strains of B. subtilis possess a quorum sensing mechanism that consists of the perception of cell density. It means that it allows the bacteria to act in a coordinated manner, giving the characteristic of survival by helping them to maintain itself in nature, since it responds to environmental conditions due to nutrient availability. This mechanism is due to peptides that control the expression of genes involved in sporulation.
Other research in the field of viability of endospores are reported by Raisman and González (2013), who refer that strains of Bacillus sphaericus and Bacillus permians under certain conditions, the viability of spores is so prolonged, that it is possible to consider that they can survive indefinitely.
The existence of many methods to conserve viability of biological products is reported worldwide. Cryopreservation is among these methods, which is adequate and is, after the lyophilization method, the most convenient to guarantee bacteria viability over time (Bagatolli 2017).
De Araujo (2016) referenced that microencapsulation under the spray drying technique is an alternative to maintain the integrity of probiotic strains. Their studies report a 72% and 70% of survival of bacilli and lactobacilli, respectively.
Gutiérrez (2016) defined that one of the most used technique in the industry to preserve biological products, due to its high reproducibility and economy, is spray drying. This author, when microencapsulated strains of Bacillus megaterium, Bacillus sphaericus and Bacillus polymyxa; Lactococcus lactis and Lactobacillus delbruecki sub bulgaricus, achieved viability for 30 days of storage.
pH dynamics. The results of the pH performance are shown in figure 1. For the three additives, the 7th-day pH value is observed as low with respect to its initial value at the two temperatures [room (25 ± 3 ºC) and refrigeration (4 ± 8 ºC)] and from there, it remains stable until the end of sampling.
The literature that deals with the subject of probiotics states that one of the characteristics of additives with cultures of Bacillus spp. is to favor the increase of lactobacilli. Therefore, in this result, the presence of lactic acid bacteria (LAB) could have an impact. Also the production of enzymes or some type of secondary metabolite produced by the bacillus. Nguyen (2017) and Nguyen and Nguyen (2017) stated that LAB are high producers of organic acids, which lower the pH and prevent colonization by undesirable bacteria.
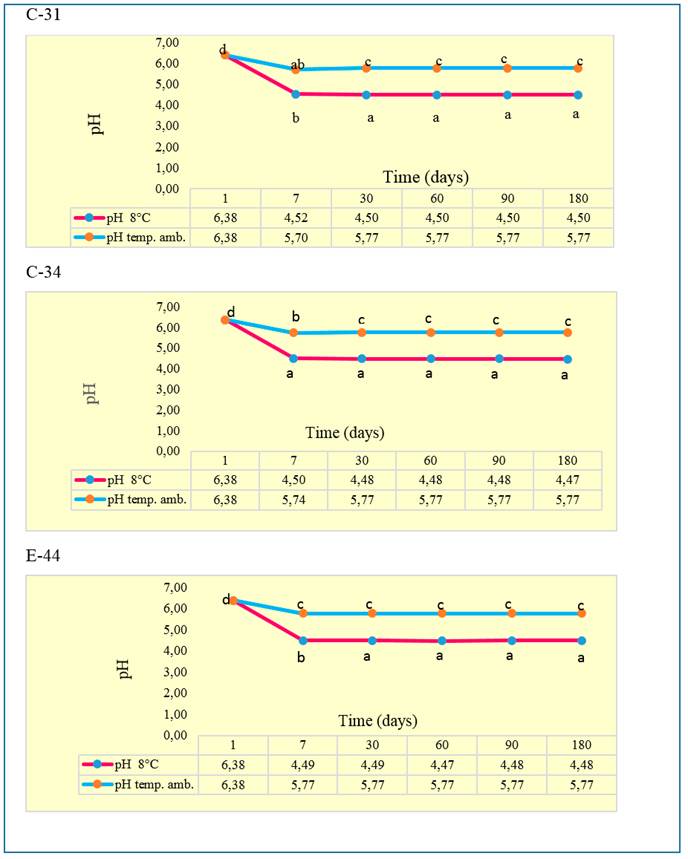
pH a,b,c different letters differ at *P<0.05 (Duncan 1955), SE±0.01
Figure 1 Dynamics of pH of the zootechnical additive SUBTILPROBIO® C-31, C-34 and E-44 at room temperature (25 ± 3 ºC) and refrigeration (4±8 ºC)
Adedeji et al. (2011) and López et al. (2013) define that pH is directly related to the degradation processes that occur during conservation. In this sense, when a biological product reaches pH values between 3.8 and 4.2, its stability is achieved. This condition causes a restriction of the activity of proteolytic enzymes and the suppression of enterobacteria and Clostridium. The evaluated product is in the range 4 and 5.
Powthong and Suntornthiticharo (2015) state that the presence of LAB, in biological products, guarantees security and stability in its use as animal feed. The LAB are microorganisms that have diverse applications and one of the most important is the biopreservation and quality of sensory characteristics of food. Those facts referred by these authors are corroborated with the results obtained by Milián et al. (2013), when evaluating the zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44 in in vivo microbiological indicators in EB24 broilers. They obtained increases in the counting of Lactobacillus spp. and decrease in coliforms at the level of caeca at 21, 35 and 42 days of sampling.
Research reported by Rendó et al. (2014) refer that, when the biological product obtained is adequate, sugars in the medium are mainly converted to lactic acid and acetic acid, responsible for the rapid fall of pH, which inhibits the growth of pathogenic microorganisms that cause great losses of biological foods for animals. The results of this research allow to correlate the previously mentioned facts, since the culture medium used for the elaboration of zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44, was formulated with final molasses as carbon source, which provides glucose, fructose and sucrose (Milián et al. 2017b).
The results obtained throughout the research are in line with the reports of Pérez et al. (2011) when they evaluated the stability in time of PROBIOMEX®, product of competitive exclusion based on lactic bacteria and Bacillus spp., with favorable results and similar to those obtained in this research. This mixture showed a high growth capacity and stability during storage for a period of 30 days under the same conditions of refrigeration and environment.
Similar results were obtained by García et al. (2013), when they evaluated the zootechnical additive PROBICID and demonstrated that it can be used and stored at 30ºC for up to 6 months.
The results of this research show the real possibility of the zootechnical additives SUBTILPROBIO® C-31, C-34 and E-44 for their conservation in both temperatures up to 180 days, since there have no biological or chemical negative effects that interfere in their quality.











 text in
text in 


