INTRODUCTION
Fungal contamination in food and feed cause significant economic losses in primary agricultural yields, in the transforming industries and in livestock farms (Godfray et al. 2016). The main contaminant fungi (molds), belonging to several ubiquitous genera, are characterized by the production of large masses of conidia easily spread by air to the soil, water, plants and animals (Adhikari et al. 2004). Fungal contamination of the raw materials also occurs during pre-harvest (field-produced fungi) and post-harvest periods, such as storage and transformation processes (storage-produced fungi) (Krnjaja et al. 2008 and Whitlow et al. 2010). Contaminating fungi prejudice the preservability and the nutritional value of food and feed by the production of lithic enzymes and pose a potential risk for consumer health by mycotoxigenic activity (McNeil et al. 1984).
The cellulolytic activity is widely distributed in the contaminating fungi belonging to Phylum Ascomycota as in the genera Bulgaria, Chaetomium, Helotium, Neurospora, Aspergillus, Cladosporium, Fusarium, Geotrichum, Myrothecium, Paecilomyces, Penicillium and Trichoderma (Lynd et al. 2002 and Tian et al. 2009). They dominate, both in abundance and in activity, the microbial community responsible for the decomposition of cellulose residues (Wilson, 2011).
Among these, species belonging to the genus Aspergillus are able to produce a large variety of glucanases that allow the complete degradation of cellulose.
Moreover, species belonging to genera Aspergillus, Fusarium and Penicillium, able to produce dangerous mycotoxins, can cause metabolic disorders resulting in biological effects on animals as liver and kidney toxicity, central nervous system effects and estrogenic effects (Greco et al. 2014). Some contaminating mycotoxin due secondary contamination in humans via eggs, meat or milk (carry-over effect), with acute and chronic toxic effects (Volkel et al. 2011).
In particular, the contamination of feeds with fungi and their spores is worldwide described. In tropical regions Aspergillus spp. predominate in several feeds and Penicillium, Fusarium and Alternaria species are recurrent contaminants of kernels and other grains (Prasad et al. 2016). In Sicily, recent studies on local row materials and feeds shown low mycotoxins contamination levels, if compared to imported products, while very few are the data on the level of fungal colonization (Finoli and Vecchio, 2003, Gallo et al. 2008 and Russo, 2015).
The aim of this study was to evaluate, in Sicilian raw materials and livestock feeds, the total fungal contamination and to detect the percentage of the three potential mycotoxigenic genera (Aspergillus, Fusarium and Penicillium). Moreover, the most recurrent Aspergillus isolates, identified at level of specie by morphological and molecular methodologies, was tested to evaluate their cellulolytic activity.
MATERIALS AND METHODS
Sampling. A total of fourteen feed samples (table 1) were collected in a feed mill located in the province of Palermo (Sicily, Italy) following the standard methodologies (Reg. CE 1441/2007; Reg. CE 401/2006; DM 20/04/1978). Aliquots of 600 g were randomLy taken from different parts of the bag or container of each feed both from packaging and storage areas. The samples were placed in sterile plastic bags, transported to the laboratory and stored at 4 °C until further analysis.
Table 1 Feed samples collected in a feed mill in the province of Palermo
| Code | Sample | Composition | Sampling area |
|---|---|---|---|
| 1 | Flaked broad bean | Raw material | Packaging area |
| 2 | Oat | Raw material | |
| 3 | Poultry feed | Soy flour, cornmeal, maize, wheat bran | |
| 4 | Flaked maize | Raw material | |
| 5 | Swine feed | Maize, wheat bran, barley, sunflower flour, carob, citrus peel | |
| 6 | Horses feed (West Performance) | Wheat bran, broad bean, flaked barley, carobs, maize, oat flour, molasses | |
| 7 | Cattle feed | Maize, barley, carobs, broad bean | |
| 8 | Poultry feed | Soy, corn, wheat bran, maize | Finished product storage area |
| 9 | Horses feed (West Performance) | Wheat bran, broad bean, flaked barley, carobs, maize, oat flour, molasses | |
| 10 | Flacked broad bean | Raw material | |
| 11 | Cattle feed | Maize, barley, carobs, broad bean | |
| 12 | Flaked maize | Raw material | |
| 13 | Horses feed (Superior House) | Flaked broad bean, flaked maize, flaked barley, flaked oat, flaked corn, sunflower flour | |
| 14 | Ruminants feed | Broad bean, barley flour, maize, wheat bran |
Fungal contamination. Isolation and enumeration of fungal colonies were carried out using serial dilution and spread plate technique (Maina et al. 2016). All samples were ground with a mill up to 0.25 mm and 1 g of each sample was homogenized in 10 mL of distilled sterilized water. Samples were analyzed in triplicate. Aliquots (0.1 mL) of ten-fold serial dilutions were inoculated in Sabouraud Dextrose Agar (SAB) and Potato Dextrose Agar (PDA) in order to evaluate the medium efficiency (the highest number of colonies). All plates were incubated at 22 °C for 9 days and the total fungal count (CFU/g) and relative percentage of Aspergillus, Fusarium and Penicillium colonies were evaluated under stereomicroscope every 3 days. Single colonies of Aspergillus spp. were sub-cultured into PDA and monoconidial pure cultures of each strain were obtained and used for further characterization. All the isolates were maintained into PDA agar slants and cryopreserved in 15% glycerol at -80 °C.
Morphological identification of Aspergillus species. Macro-morphological features of the Aspergillus isolates were determined in PDA, SAB and Czapek dox Agar (CZ) according to Lin and Dianese (1976) and Thathana et al. (2017). For each Aspergillus isolate, plugs from the edge of 7-day-old pure colonies were taken by a sterile 3 mm cork borer and placed singly at the center of 10 cm diameter plates with the three different media, in three replicates. The plates were incubated at 25°C for 9 days and diameter, growth rate, color (conidia and reverse), shape and texture of each colony were recorded every 3 days. Microscopic observations were performed at the microscope Axioskop (Zeiss, Oberkochen, Germany) coupled to an AxioCam MRc5 (Zeiss, Oberkochen, Germany) digital camera. Images were captured using the software AxioVision 4.6 (Zeiss, Oberkochen, Germany). The microscopic features were conidial heads, vesicle shape and diameter, presence of metulae and size and shape of phialides and conidia (30-50 measurements) (table 3). The identification was carried out using taxonomic keys (Barnett and Hunter 1972, von Arx 1981, Cole and Kendrick 1989, Pitt and Hocking 1999 and Klich, 2002).
DNA extraction, PCR and sequencing. Genomic DNA was extracted from pure colonies of the most recurrent Aspergillus isolates following the CTAB-based method (Torta et al., 2015). The DNA was suspended in 100 µL of TE 1x (0,121g of Tris 10 mM and 0,037g of EDTA 1mM in 100 mL of distilled water) quantified by using NanoDrop ND-1000 and stored at -20°C. The primers ITS1F (Gardes and Bruns 1993) and ITS4 (White et al. 1990) were used for the amplification of the ITS1-5.8S-ITS2 region. The PCR assay was performed in a total reaction volume of 25 µL consisting of PCR buffer 10X (Thermo Scientific), 0,2 mM of each dNTPs, 0,3 µM of each primer, 0,5 U of Taq DNA polymerase (Dream Taq, Thermo Scientific) and 1 µL of target DNA. The amplification was carried out in a MultiGene OptiMax thermocycler (Labnet International Inc.) with an initial denaturation cycle at 94 °C for 3 min followed by 35 cycles at 94 °C for 30s, annealing at 55 °C for 30s, elongation at 72 °C for 45 s, with a final extension at 72 °C for 10 min.
PCR products were separated by electrophoresis in 1% agarose gel and amplicons were detected under UV transilluminator (330 nm).
PCR products were purified using Exo I-SAP protocol according to the manufacturer’s instructions (Applied Biosystems, Foster City, CA). Primer ITS1F was used in the sequencing reaction. Sequencing reactions were performed with BigDye Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA) followed by Ethanol/EDTA/Sodium Acetate precipitation (according to manufacturer’s instruction). Finally, capillary electrophoresis was performed in the 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA). The sequences were aligned against those already deposited in the GenBank databases using BLASTn tool (Altschul et al., 1990). New sequences were deposited in GenBank.
Qualitative determination of the cellulolytic activity. The cellulolytic activity of the Aspergillus isolates was performed according to Mandels et al. (1976) and Ghorbani et al. (2015). The strains were grown in 10 mL of Mandels liquid medium (Mandels et al., 1976) in tubes containing a 1x6 cm Whatman No 1 paper strips and incubated at temperatures of 25 and 30 °C under static condition, in order to evaluate the effect of temperature on the cellulolytic activity. The control was tubes not inoculated. Cellulolytic activity was also evaluated in shaking condition (90 rpm) at 30 °C. After 5, 10, 15 and 21 days, fungal growth and paper maceration were evaluated by using a rating scale from 0 to 5 (0 = no fungal growth and no maceration; 5 = complete paper colonization and maceration).
Statistical analysis. Data on total fungal contamination were subjected to analysis of variance (ANOVA) through the Statgraphics Plus 5.1 program. The averages were compared by the Fisher LSD multiple comparison test (P ≤ 0.05).
RESULTS
Fungal contamination of feed samples. All feed samples showed fungal contamination, and total fungal count varied within the samples and also in relation to culture medium (figure 1). At the third day of incubation, on PDA, fungal colonies were observed only on sample 1 (flaked broad bean), while on SAB they were present in 12 out of 14 feed samples. At 6 days number of fungal colonies increased in all the samples both on SAB and PDA. These values remained fairly constant until the ninth day but in PDA total fungal population was higher than in SAB. Total population ranged from 1.11x106 to 1.31x108 and from 1.11x103 to 1.58x106 CFU/g on PDA and SAB respectively. Statistically significant differences were observed at nine days of incubation within the feed samples on both media, although total fungal contamination was similar in the two sampling areas (table 2). Oat (sample 2) and poultry feed (sample 8) showed the highest level of total fungal contamination on PDA and on SAB respectively (table 2). Regarding the potential mycotoxigenic fungal genera, colonies belonging to Aspergillus, Fusarium and Penicillium were obtained on 3 (samples 1, 3, 12, on PDA) and on 4 (1, 3, 11, 12 on SAB) out of 14 samples. The frequency of the three isolated genera is shown in figure 2. In general, Fusarium sp. was the prevalent species isolated (from 10 and 7 samples, respectively on PDA and SAB). Penicillia, isolated from 5 and 4 samples on PDA and SAB respectively shown the lower frequency. Nevertheless, the percentage of the three genera varied depending on the culture medium. Aspergillus spp. colonies were more frequently isolated on SAB, while Fusarium spp. and Penicillium on PDA. With regard to total fungal population, the frequency of isolation of Aspergillus colonies was about 10% on both media (data not shown), whereas in each sample the percentage ranged from 14 to 36% and from 1.33 to 70% on PDA and SAB respectively.
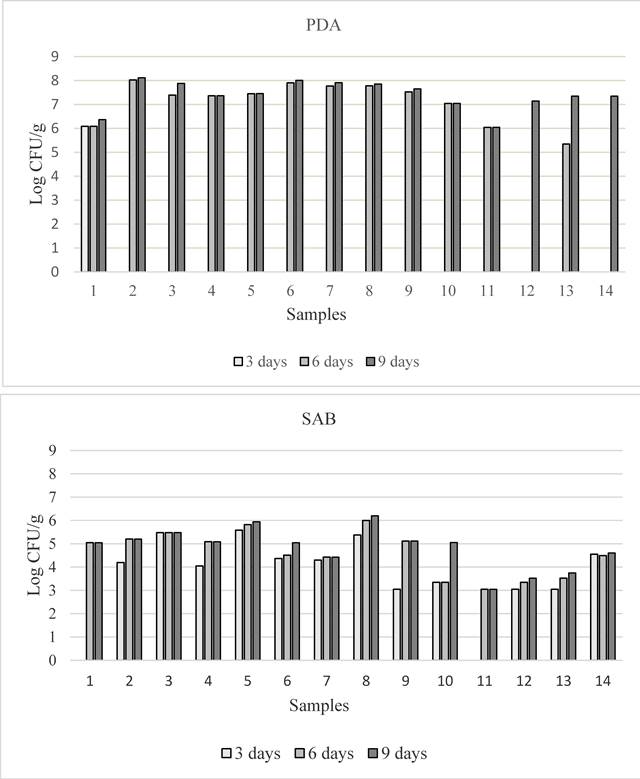
Figure 1 Total fungal contamination detected in the 14 feed samples at 3, 6 and 9 days of incubation, on PDA (above) and SAB (below)
Table 2 Total fungal contamination in the analyzed feed samples at 9 days of incubation on PDA and SAB, expressed in Log of UFC/g and relative percentage of Aspergillus colonies.
| SAMPLE | PDA | %Aspergillus | SAB | %Aspergillus |
|---|---|---|---|---|
| 1= Flaked broad bean | 6.37 + 2.28 a | 0 | 5.05 + 1.84 ab | 0 |
| 2= Oat | 8.12 + 0.39 c | 25 | 5.20 + 0,30 ab | 59 |
| 3= Poultry feed | 7.88 + 0,40 abc | 0 | 5.48 + 0.22 ab | 0 |
| 4= Flaked maize | 7.37 + 0.46 ab | 36 | 5.09 + 1.85 ab | 0 |
| 5= Swine feed | 7.46 + 0.41 ab | 31 | 5.94 + 0.08 bc | 3 |
| 6= Horses feed | 8.01 + 0.88 bc | 0 | 5.05 + 1.74ab | 0 |
| (West Performance) | ||||
| 7= Cattle feed | 7.91 + 0.17 bc | 18 | 4.43 + 0.09 a | 8 |
| 8= Poultry feed | 7.85 + 0.14 abc | 14 | 6.20 + 0.26 c | 1.33 |
| 9= Horses feed | 7.65 + 0.09 ab | 20 | 5.12 + 0.52 ab | 70 |
| (West Performance) | ||||
| 10= Flacked broad bean | 7.05 + 2.50 a | 0 | 5.05 + 1.63ab | 66 |
| 11= Cattle feed | 6.05 + 2.17 a | 0 | 3.05 + 1.17a | 0 |
| 12= Flaked maize | 7.15 + 0.59 a | 0 | 3.52 + 1.22a | 0 |
| 13= Horses feed | 7.35 + 0.66 ab | 0 | 3.74 + 1.30 a | 60 |
| (Superior House) | ||||
| 14= Ruminants feed | 7.35 + 2.50 ab | 0 | 4.60 + 1.54a | 0 |
abcdIn each column, values followed by same letters are not statistically different according to Fisher LSD Test (P≤0.05).

Figure 2 Frequency of Aspergillus, Fusarium and Penicillium isolated at 9 days of incubation on PDA (above) and SAB (below) from 14 feed samples
Identification of Aspergillus spp. Aspergillus spp. were isolated from 8 out of 14 feed samples and eight isolates belonging to Section Nigri, Section Flavi and Section Nidulantes, were selected on the basis of the frequency of isolation and of the macro-morphological features. The strains belonging to the Section Nigri presented colonies with dark-brown to black colour and reverse pale or light yellow color; the conidiophores bearing spherical vesicles were uniseriate or biseriate producing globular or subglobular conidia (smooth, finely rough or rough). Isolates belonging to the Section Flavi were characterized by typical yellow-green mature conidia, reverse light yellow to yellow colour; mainly uniseriate conidiophores with globular vesicle producing globular conidia (smooth or finely rough). Isolates of Section Nidulantes presented colonies with pale yellow or yellow colour and reverse yellow or pale orange color; microscopically they presented uniseriate conidiophores, subglobular vescicles, subglobular rough spores and characteristic Hulle’s cells. Based on morphological characteristics, the isolates were identified as Aspergillus amstelodami (L. Mangin) Thom & Church (figure 3), Aspergillus awamori Nakaz., Aspergillus flavus Link., Aspergillus niger Tiegh., Aspergillus oryzae (Ahlb.) Cohn. and Aspergillus tubingensis Mosseray (table 3).
An amplicon from about 500 to 600 bp of the ribosomal region including the two non-coding ITS1 and ITS2, and the 5.8S rDNA gene was amplified from 8 Aspergillusspp. isolates. Apergillus spp. isolates had high match with published sequences in GenBank showing maximum identities of 99-100% with Apergillus sections Nigri (A. awamori, A. niger, A. tubingensis), Flavi (A. flavus, A. oryzae) and Nidulantes (A. amstelodami) (table 3).
Table 3 Morphological features of the 8 Aspergillus spp. isolated from feed samples. T= teleomorph (sexual reproductive stage); A= anamorph (asexual reproductive stage)
| Isolates | Feed samples | Colour on PDA | Colony growth at 25°C (mm) | Stage | Conidiophores | Conidia shape | Conidia size (µm) | Vesicle size (µm) | Hulle’s cell | Species | GenBank Accession number | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PDA | SAB | CZ | ITS | ||||||||||
| SAAF 7 | 5 | Black | 55x57 | 60x65 | 66x67 | A | Biseriate | Rough | 4.6-5.7 | 55-70 | - | A. niger | MK503962 |
| SAAF 12 | 8 | Black | 83x83 | 85x85 | 85x85 | A | Biseriate | Rough | 4.5-6 | 25-40 | - | A. niger | MK503964 |
| SAAF 15 | 9 | Black | 79x81 | 80x82 | 83x84 | A | Biseriate | Rough | 4.6-5.8 | 30-45 | - | A. niger | MK503966 |
| SAAF 14 | 7 | Dark-brown | 35x36 | 32x33 | 45x47 | A | Biseriate | Smooth | 3.5-4.1 | 20-40 | - | A. tubingensis | MK503965 |
| SAAF 10 | 12 | Black | 80x80 | 85x85 | 85x85 | A | Biseriate | Smooth | 4-6 | 40-50 | - | A. awamori | MK503963 |
| SAAF 4 | 7 | Green | 67x70 | 75x75 | 83x83 | A | Biseriate | Smooth -finely rough | 3.2-5.8 | 18-36 | - | A. flavus | MK503960 |
| SAAF 17 | 10 | Yellow-green | 75x75 | 80x80 | 75x75 | A | Biseriate | Smooth | 5.3-7.2 | 25-40 | - | A. oryzae | MK503967 |
| SAAF 6 | 2 | Yellow | 25x25 | 25x25 | 30x30 | T | Uniseriate | Rough | 4.3-6.5 | 20-25 | + | A. amstelodami | MK503961 |
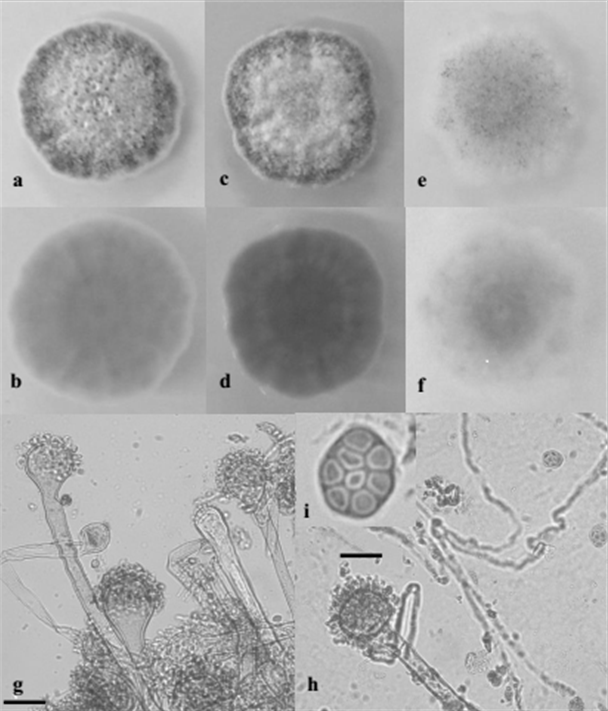
Figure 3 Macroscopic and microscopic features of A. amstelodami SAAF 6. 10 days- old colonies grown on PDA (a = F; b = R), SAB (c = F; d = R) and CZ (e = F; f = R); g, h ) Conidiophores, vesicles and conidial head; i) ascus and ascospores. F = Front, R = Reverse. Bar: g, h, = 25 µm; i = 10 µm
Qualitative determination of the cellulolytic activity. All Aspergillus strains showed cellulolytic activity, growing on the filter paper (table 4). In all the control tubes the filter paper was not altered. Strains A. niger SAAF 7, A. awamori SAAF 10 and A. tubingensis SAAF 14 shown the highest cellulolytic activity, completely macerating the paper at the end of the test. On the other hand, the temperature seems affects the cellulolytic capacity. At 25 ºC the filter paper was generally less macerated, whereas A. amstelodami SAAF 6 showed cellulolytic activity only at this temperature. Agitation induced greater or lesser maceration of the filter paper depending on the Aspergillus strain.
Table 4 Cellulolytic activity of eight Aspergillus strains, detected up to 21 days after inoculation at 25 and 30 °C in static (S) and shaking (Sh) condition.
| Strains | 25 ºC S | 30 ºC S | 30 ºC Sh | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 21 | 5 | 10 | 15 | 21 | 5 | 10 | 15 | 21 | |
| A. amstelodami SAAF 6 | 1 | 1 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A. awamori SAAF 10 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 3 | 5 |
| A. flavus SAAF 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| A. tubingensis SAAF 14 | 1 | 1 | 2 | 2 | 0 | 1 | 3 | 5 | 1 | 1 | 3 | 5 |
| A. niger SAAF 7 | 1 | 1 | 1 | 1 | 1 | 3 | 4 | 5 | 1 | 1 | 3 | 5 |
| A. niger SAAF 12 | 1 | 2 | 3 | 4 | 1 | 1 | 2 | 2 | 0 | 1 | 2 | 4 |
| A. niger SAAF 15 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 3 | 1 | 1 | 1 | 1 |
| A. oryzae SAAF 17 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 2 | 4 |
DISCUSSION
In this study, for the first time, fourteen feed samples collected in two sampling area in a mill in Sicily were monitored to detect the presence of contaminating fungi. The level of fungal contaminations in all feed samples ranged from about 3 to 8 Log CFU/g with the recurrent presence of colonies belonging mostly to the ubiquitarious genera Aspergillus, Fusarium and Penicillium, the main toxigenic molds. Between them Fusarium was the predominant genus isolated from samples, followed by Aspergillus and Penicillium, according with the results of other studies conducted in Italy and Europe (Chadd 2004, Covarelli et al. 2011 and Gregori et al. 2013). Moreover, the culture medium efficiency was also tested revealing differences in the estimation of fungal populations on the two utilized agar media. The universal medium PDA, ensuring the isolation of the highest number of fungal colonies, allow to assess the total fungal population, while to evaluate the Aspergillus population SAB should be preferred (Krnjaja et al. 2008). Regarding the two sampled area ours data showed no significantly differences, but the levels of total fungal contamination and above all the presence of toxigenic fungi revealed inappropriate techniques during the feed producing chain, indicating a low quality and bad treatment of the materials.
Usually, the population of these fungal contaminants in feed and food is strictly related to the relative concentrations in mycotoxins, in particular in bad-stored or long-exported materials (Dalcero et al. 1998, Krysinska-Traczyk et al. 2001, Gonzalez Pereyra et al. 2012; and Greco et al. 2014). On the contrary, in Sicilian raw materials and finished livestock feeds, despite the presence of these fungal contaminants, previous studies indicate that the dangerous metabolites (afla-, ochra- and Fusarium-toxins) resulted to be absent or detected at low level (Finoli and Vecchio 2003, Gallo et al. 2008 and Russo 2015). However, high level of fungal contaminants cause in feed and food the loss of nutritional value, due to their degradation by enzymatic activity (Driehuis and Oude Elferink 2000, Megan et al. 2003, andAmigot et al. 2006).
Among the contaminating fungi, some species within the genus Aspergillus are the most critical for their mycotoxigenic and cellulolytic activity, causing a potential risk for animal and human health and for the degradation of trophic substance (Hanif et al. 2004 and Patyshakuliyeva 2016). The production of cellulolytic enzymes by Aspergillus strains isolated from analysed feed samples was qualitatively evaluated. A. niger, A. tubingensis and A. awamori showed the highest expression level of cellulase enzymatic complex. On these basis, oat and poultry feed, the most contaminated livestock tested feeds, may have lost most of their organoleptic and nutritional quality.
As presented in the results, the highest cellulolytic activity is shown in the Aspergillus species that are part of the "Nigri section", in this case A. niger, A. awamori and A. tubingensis, the most used at the industrial level. These fungi, characterized by a high distribution worldwide, are also considered the most common fungi that occur in the decomposition of food (Raper and Fennell 1965) by the production of a great variety of enzymes, such cellulases, xylanases, proteases and phytases, mainly, and also α-amylases, pectinases, amyloglucosidases and lactases (Krishna 2005 and Aguiar, 2010). As regards the effect of temperature on the cellulolytic activity of microorganisms, a greater degradation rate was observed for most of the cases at 30ºC. It is necessary to point out that temperature is one of the main factors that affect the biomass yield, an aspect that is closely related to the type of microorganism that is being tested. Each type of microorganism has a certain optimum growth temperature where it expresses its highest productivity. Temperatures close to this can also have a similar effect. Several authors propose optimum temperatures for the growth of different species of Aspergillus between 25-35ºC (Passamani et al., 2014). On the other hand, temperature affects not only the growth of the biomass but also the production of different metabolites. Several authors have reported optimal temperatures for the production of cellulases in Aspergillus species between 30-35ºC (Bastawde 1992, Velkovska et al. 1997, Shahriarinour et al., 2011 and Saithi et al. 2016).
On the other hand, once the microorganisms have secreted their enzymes into the media, the enzymatic activity is conditioned by different factors such as pH, ionic strength, temperature, among others (Seager et al. 2016). These factors condition an optimal range of activity that varies depending on the type of enzyme. Although, in relation to temperature, an increase of this brings an increase in the speed of the reaction and consequently in the enzymatic activity (Voet et al. 2016). Regarding to the effect of the agitation, it is observed that at the end of the 21 days of experimentation all the strains with agitation, except in the case of A. niger, had a higher cellulolytic activity respect to those without agitation. This is mainly due to the fact that the agitated systems allow a greater interaction of the microorganism with the substrate, which allows a better colonization and use of the specific substrate surface that results in a greater production of enzymes. (Jeong et al. 2006).
In regard to the mycotoxigenic activity (not evaluated in this study), among the six identified Aspergillus species, A. niger and A. flavus are reported as agents of human and animal mycotoxicosis, the first associated with the production of ochratoxin A, and the second with aflatoxins B1 and B2 (Yu, 2012). However, not all the strains of these species are able to metabolize dangerous mycotoxins. As matter of fact, it is known that in A. flavus species only about 40-50% of strains produce aflatoxins and only about 20% of A. niger strains are ochratoxigenic (Abarca et al. 2001 and Davari et al. 2015). Although the detection of toxigenic fungi in the analyzed samples not necessarily indicate that mycotoxins are naturally occurring in the feed, it alerts to the potential risk of contamination. Other species isolated from feed samples, such as A. awamori and A. oryzae, not associated with the production of toxic metabolites, are largely used in food biotechnologies (Siedenberg et al. and 1998; Takagi 2014).
CONCLUSIONS
This first study on the evaluation of the level of fungal contamination in Sicilian raw materials and livestock showed the presence of Aspergillus spp., Penicillium spp. and Fusarium spp, in the sampled materials. In particular, Apergillus sections Nigri (A. awamori, A. niger, A. tubingensis), Flavi (A. flavus, A. oryzae) and Nidulantes (A. amstelodami) were identified by morphological and molecular methodologies. The production of cellulolytic enzymes was observed in all the Aspergillus strains, but A. niger SAAF 7, A. awamori SAAF 10 and A. tubingensis SAAF 14 shown the highest degradation activity.
On the basis of these results it is assumed that the most contaminated feed by these fungi can be the most degraded from a nutritional point of view.
This study highlights the importance of continued monitoring and control of fungal contamination in feed and food. Strategies aimed to prevent them in field, during storage and in all the feed production chain should be implemented. The control of this fungal contaminants and corrects techniques of animal feed production, in fact, can assure not only high level of animal’s health but also high quality level of feeds both in palatability and nutritional values.











 text in
text in 


