INTRODUCTION
The medicinal potential of the genus Sapindus L. has been studied by authors from several countries and within this genus, the species Sapindus saponaria L. reflects its application in the treatment of various diseases. The plant has been used for curative purposes in countries like Egypt, Kenya, USA, Peru, Brazil, Puerto Rico and Cuba. The genus Sapindus consists of about 12 species of trees or shrubs distributed throughout the tropics and subtropics of the world. In the Caribbean basin and South América it is represented by Sapindus saponaria L. and other species (Suhagia B, et al 2011, Abreu O, 2005, Gauthier C, et al 2009, Man S, et al 2010, Koczurkiewicz P, et al 2015, Jayadev R, et al 2004).The known detersive properties of these species are conferred by the saponins contained in the pericarp of the fruit, and other parts of the plant, which can also exert a broad biological and pharmacological activities: immunoadjuvants for human and veterinary vaccines (De Groot C, 2016), anti-oedematous, venotonic, bronchiolitic, hypocholesterolhemic, anti-inflammatory, cytotoxic activity against several neoplasms, antimicrobial, hepatoprotective, insecticidal, piscidal, spermicidal.(Alhosin M, 2011, Ospina L et al 2013, Sharma A, et al 2011). Since ancient times, the etnomedical knowledge of herbs, natural products and spices have been used for preventing several diseases, including cancer (Abreu O, 2005, Man S, et al 2010, Koczurkiewicz P, et al 2015). Prostate cancer (PC) is among the most frequent metastatic malignancies contributing to cancer deaths in males. PC is the second most common cancer and the sixth leading cause of cancer death among men worldwide, with an estimated recorded amount of 1.1 million cases and 307 000 deaths in 2012 (Sadeghi-Gandomani HR, et al 2017). Prostate cancer is the second most commonly occurring cancer in men and the fourth most commonly occurring cancer overall. There were 1.3 million new cases in 2018 (Bray F, et al 2018). With 5291 new cases of prostate cancer, in 2018, in Cuba this location ranks in general, second, after lung cancer, with 6914 new cases in the same year, that is only 1653 new cases less in 2018 as difference between the two locations. While in men, prostate cancer ranks first with 21.3 % of all new cases, leaving new lung cases in second place with 16.8 % of new cases in 2018 (Bray F, et al 2018). Thus, developments of innovative therapies for the prevention, diagnosis and treatment of prostate cancer are far more than needed.
Studies from the last years confers to saponins of Sapindus saponaria L. a good in vitro cytotoxic activity against cancer cell lines Hela (cervix human carcinoma), WiDr (colon carcinoma) and KB (oral carcinoma) with very small concentrations of these saponins
The objectives of the present study are to evaluate different parts of the plant S. saponaria L., as a source of saponins by measuring quantitatively the hemolytic activity of aqueous extracts of fruits, seeds and stems and to explore their in vitro antiproliferative potential in the cell line of human prostate cancer PC3.
MATERIALS AND METHODS
Plant material and extraction
The fruits, seeds and stem fragments of Sapindus saponaria L. were collected in Manabí, Latitude: 0o 50´51, 36” S; Longitude: 80o 9´49, 79” W; at an average Altitude of 86 meters above sea level. Ecuador. The plant material (fruits, seeds and stem bark) of Sapindus Saponaria L. was collected in the morning between 10:30 a.m. and 11:00 a.m. at a temperature between 25 and 30 °C during the month of November. Repeated experience 3 consecutive years, yield abundant samples as well as validated collection-handling protocols. The arborization process is registered and Good Laboratory Practices were used for the transport and conservation of the samples. The registration code for fruits and stems is 2013XII15 Ss01/F and 2013XII15 Ss01/T respectively. The parts affected by insects or with signs of damage were discarded and the rest were fractioned with the help of a scissor and a press in the case of the seeds, for its greater hardness. The plant material separated into fruit, seed and bark of the stem; was washed with plenty of running water to remove dirt and then performed a final wash with distilled water. After dried on filter paper at 25 oC, finally the mass was determined using an analytical balance (Sartorius AG, China).
For the preparation of the extracts, 29.93 g were weighed in the cases of fruit and seed and 34.39 g of stem bark, in parallel a volume of 1000 mL of distilled water was taken to the boil, taking 29.93 mL in the case of the fruit and 34.39 mL in the case of the bark and proceeding to make an infusion with both the fruit and the bark of the stem. For the seed a volume equal to 250 mL of distilled water was taken and a decoction was performed until reaching a final volume of 29.93 mL.
The extracts obtained were lyophilized and stored at 4 - 8 0C to perform the biological activity assays and validation repetitions.
Hemolysis assay
The quantitative determination of saponins in the extracts was performed by the modified technique “Red Blood Cell Test System” (Pape W, 1992).
A fresh calf blood sample was obtained from the slaughterhouse. Red blood cells (RBC) were washed and centrifuged several times, to remove white cells and any traces of plasma. Finally, a RBC suspension containing about 8 x 109 cells was obtained.
Several aliquots of extracts and reference saponins solution (10.0, 20.0, 30.0, 40.0, 50.0, 60.0, 70.0, 80.0 µL) were added into reactions vials. Later, each reaction vial was filled up to 975.0 µL with PBS and was incubated with 25.0 µL of RBC suspension for 10 min, with constant shaking, at room temperature. The incubation period was terminated by rapid, high-speed (10 000 r.p.m) centrifugation for 1 min. This removes intact cells and debris from the medium after centrifugation. The resulting supernatant was then monitored spectrophotometrically at 540 nm against blank. The blank contained the extract sample diluted in PBS only.
Spontaneous hemolysis was monitored by adding 25.0 µL of RBC suspension to 975.0 µL PBS. This gives the zero hemolysis value, whilst 25.0 µL of RBC suspension is added to 975.0 µL distilled water to give the 100 % hemolysis value.
A standardized concentration curve of a reference saponins Quillaja saponaria Molina (0.5 µg/mL) was applied against hemolyzed extract fractions in which the values of the extracts were interpolated to obtain their concentration of saponins.
Antiproliferative assay
PC 3 (human prostate adenocarcinoma, grade IV, ATCC® CRL1435™) cells were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). Cells were cultured in RPMI (Hyclone, USA) medium supplemented with 10 % fetal bovine serum (PHA, Canada). PC 3 (7.5 x 10 4 cells/mL) cells were seeded in 96-well plates (Costar, USA). Cells were maintained at 37.8 0C in a humidified atmosphere of 5 % CO2 in air for 24 h at 37 °C.
After incubation, 0.2 mL of each aqueous extract and control saponins solution Quillaja Saponaria Molina was diluted to give the final concentrations of 100.0, 50.0, 25.0, 12.5 and 6.25µg/mL. A reference drug Paclitaxel was assayed. A volume of 100.0 µL of each extract, Paclitaxel drug and Q. saponaria M. was added to the wells containing PC3 culture cells. The cells were further incubated for 48 h in the presence of each agent, followed by evaluation of cell growth using the high cell density assay (Keepers Y, et al 1991). The culture medium was removed and 50.0 μL of a solution of crystal violet was added. After, the plates were dryed for 24 h at 25 °C and 50.0 µL of 10 % acetic acid were added. Finally, the absorbance was measured at 562 nm in the plate reader (Amersham, EUA).
Data analysis
The 50 % inhibitory concentration of cell proliferation (IC50) and the half-maximal hemolytic concentration (CH50) was calculated and processed by the program Calcusyn (Calcusyn, Version 2.0, 1997, Biosoft, USA). The maximal inhibition of cell proliferation and hemolytic activity were calculated and showed as percent (Microsoft Excel, version 2010).
RESULTS
Antiproliferative activity
The extracts evaluated markedly inhibited the cellular proliferation at very low concentrations. The IC-50 values of each product for cell line PC 3 are shown in Table 1. The IC50 value for fruit extract was below IC50 value of Paclitaxel drug. On the other hand, Paclitaxel and fruit extract were able to inhibit the 50 % cell growth, at very low concentration.
The morphological changes observed in PC3 tumor cell line due to the antiproliferative effect of the extract of fruit was showed in Figure 1. Untreated cells taken as a negative control formed a homogenous monolayer of characteristic morphology (Figure 1 A). Figure 1 B shows that at low concentrations of the extract, the cells changed their typical morphology. Furthermore, they showed unmistakable irregularities at cell borders, cell elongation and rupture of cell membrane. The effects of highest concentrations of the fruit extract applied are shown in Figure 1C and D, where the destruction of a part of the cell monolayer can be seen at 10 mg/mL of fruit extract, with the formation of vacuoles by binding the contents of multiple cells. In figure 1 E, F and G the effect of antineoplastic Paclitaxel, used as positive control in the antiproliferative assay is showed. Low concentrations of the control drug inhibit the formation of monolayer and change the morphology of the individual cells towards rounded and separated cells with abundant vacuoles and irregular borders.
Saponins quantification
Table 2 summarizes the concentrations of saponins present in the three extracts determined by interpolation in Q. saponaria M. curve (y=0.371x-0.44; r= 0.9388). As can be seen, the concentration of saponins from fruit extract is higher than from seed and stem extracts.
Hemolytic activity
The fruit extract exhibited markedly (88.5 %) hemolytic activity. In contrast, low hemolytic activities were achieved by seed (32.4 %) and stem (12.5 %) extracts. Figure 2 shows the concentration versus hemolytic fraction curves for the three extracts and control saponins. As can be seen, the fruit extract achieved, at very low concentration, 50 % hemolysis of erythrocytes (CH50= 1.72) and the extract least hemolytic was obtained from the stem (CH50=150.90), which is corresponding with lower saponin concentration quantitatively determined also in stem extract. Saponin control solution Q. saponaria M. exhibited higher CH50 than fruit extract.
DISCUSSION
The foaming properties, as well as hemolytic activity of extracts evaluated, are attributed to structural features characteristic of saponins and their amphiphilic nature which results from the presence of a hydrophilic sugar moiety and a hydrophobic genin, that make the physic-chemical behavior as biological detergents (Podolak I, et al 2010). This study shows that the extracts obtained from fruits, stems and seeds of S. saponaria L. exhibited potent antiproliferative effect against the cell line PC 3 taking into account the high percents of inhibition of cell proliferation indicated previously. These effects are in correspondence with the amount of saponins estimated in each of them.
The cytotoxicity of these extracts is probably due to the presence of secondary metabolites with pharmacological interest found in this species, such as saponins and flavonoids. (Suhagia B, et al 2011, Abreu, O. 2005, Gauthier C, et al 2009, Man S, et al 2010, Jayadev R, et al 2004, Orlando A, and Guirado A. 2005).
Cytotoxic and chemo preventive roles of saponins have been also discussed by others authors (Podolak I, et al 2010). The saponins are glycosides that are found at high concentrations in extracts obtained from the pericarp of the fruit of S. saponaria L. according to outcomes of hemolysis assay. This explains their high haemolytic potential and powerful antiproliferative effect. Its marked antiproliferative effect was seen in Figure 3 and it’s comparable with the cytotoxic effect observed for the reference drug Paclitaxel. The triterpene saponins are the most abundant active substances of the extracts studied in the present work. They have been related to numerous pharmacological activities of great biomedical interest. Some of these properties are very dependent on their detersive properties and biophysical actions on cell membranes, as is the case of its capability to improve the induction of the immune response and to act as a vaccine adjuvant. Other activities related to more complex mechanisms, such as cytostatic, antineoplastic, pro-apoptotic and anti-invasive effects. Especially at high concentrations of saponins (more than 100.0 μM), they exert a predominantly cytotoxic and hemolytic effect caused by the massive permeabilization and destruction of cell membranes, especially erythrocytes. As we have seen in the present work, the inhibition of cancer cells, antiproliferative activity, induction of apoptosis and attenuation of invasiveness occur even at very low concentrations where there is no destruction of the integrity of membranes. The cellular context and the factors involved in the case of in vitro techniques is excessively simplified and this whole subject of how to use saponins in the treatment of cancer is much more complex and rich as is the tissue and physiological environment where a tumor develops. The effect of saponins on cancer cells is multidirectional. They can effectively increase the permeability of the membranes of certain cells and that changes their physiology, induces changes in the apoptotic cascade, directly inhibits aspects of the cell cycle, inhibits the invasiveness, mainly due to disorganization of the cellular cytoskeleton, that is, they present a large versatility in their potential actions, some of which are very related to the target-environment (Koczurkiewicz P. et al 2015).
However, stem and seed extracts also showed high antiproliferative effect, while the concentrations of saponins detected in them by hemolysis assay were smaller in comparison with fruits. This indicates that in these extracts not only the saponins, but also other secondary metabolites may be co-responsible for the confirmed cytotoxicity.
According to phytochemical studies published by other authors, in the extracts of this species can also be found flavonoids as the luteolin (Sosa I, 1998), which has shown antiproliferative activity against prostatic tumour cell lines (Bucay L, 2009). This would explain why the extract of the fruit-pericarp was the more cytotoxic. It presents, not only elevated concentrations of saponins, but also other metabolites which improve its cytotoxicity as the results of our own group shows (Mena V L, 2015).
Saponins induce a strong adjuvant effect to T-dependent antigens as well as to T-independent antigens. Saponins also induce strong cytotoxic CD8 + lymphocyte responses and enhance the response to antigens at the mucosal level. However, saponins are surfactants and cause hemolysis of red blood cells in vitro, although hemolysis does not appear to be directly related to adjuvant activity. There are numerous works which results suggested that the adjuvant activity of saponins was not related to their hemolytic activity. It was considered that not only the functional groups themselves, but also the general conformation of said functional groups, affected the adjuvant activity of the saponins. Saponins have been widely used as adjuvants for many years and have been included in several veterinary vaccines. However, the adjuvant action of saponins was not pronounced in some of the non-mammalian species tested. More recently, some groups started to use saponins as part of more sophisticated formulations such as ISCOMS or immune stimulating complexes (De Groot C, et al 2016, Mena V L, et al 2015).
Saponin not only has stimulatory effects on the components of specific immunity, but also has some non-specific immune reactions, such as inflammation and stimulation of innate immune functions. The mechanisms of immune-stimulating action of saponins have not been clearly understood, but many explanations have been presented. It is reported that saponins induce the production of cytokines such as interleukins and interferons that could mediate their immune stimulating effects. They are likely to interact with antigen-presenting cells to induce many of these responses. The incorporation of saponins in the cellular or endosomal membranes could expose the antigen incorporated to the cytosolic proteases (De Groot C, et al 2016, Mena V L, et al 2015).
Table 1 IC50 Values of 50 % inhibitory concentration of cell proliferation and inhibition percent In (%) for Q. saponaria M., Paclitaxel and extracts of fruit pericarp, stem, and seed in the PC 3 cell line.
| Products | IC50(μg/mL) | In (%) |
|---|---|---|
|
Fruit Seed |
3.79 90.66 |
92 74 |
| Stem | 81.77 | 76 |
| 17.75 | 89 | |
| Paclitaxel | 3.98 | 94 |
Inhibition percent (In), 50 % inhibitory concentration of cell proliferation (IC50).
Table 2 Concentrations of saponins in the aqueous extracts of fruits, seeds and stems of S. saponaria L.
| Extract |
Saponin concentration (µg/mL) ± SD* |
|---|---|
| Fruit | 757.0±1.34 |
| Stem | 30.5±0.13 |
| Seed | 173.0±1.11 |
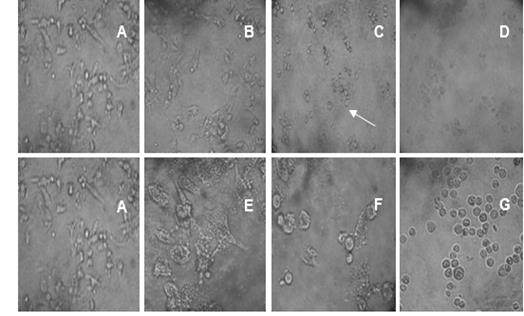
Fig.1. Antiproliferative effect of S. saponaria L. fruit extract and Paclitaxel at different concentrations on the PC3 cell line. A) Control cell line; homogeneous monolayer with characteristic morphology. B) 0.01 µg/mL of fruit extract. C) 0.1 µg/ mL of fruit extract. The arrow indicates the vacuoles. D) 10.0 µg/mL of fruit extract. E) Paclitaxel concentration (0.1708 µg/mL), F) Paclitaxel concentration (1.708 µg/mL), G) Paclitaxel concentration (17.08 µg/mL).
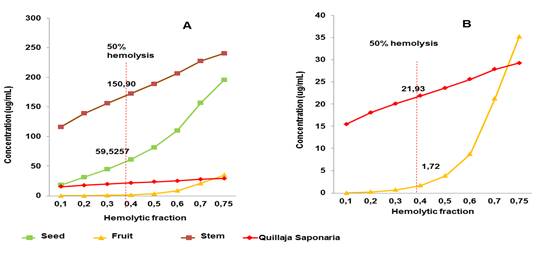
Fig. 2 A) Concentration curves vs. Hemolytic activity for the three extracts and saponins control. B) Concentration curves vs. Hemolytic activity which shows that both the saponins control and the fruit extract achieved, at very low doses, 50 % hemolysis of erythrocytes. The red line indicates, in both graphs, the concentration at which 50 % hemolysis (CH50) is achieved.
CONCLUSIONS
Summarizing, the biological activity showed by Sapindus saponaria L. extracts in this study, all three extracts contain saponins at different concentrations, showing a good cytotoxic activity against PC3 cell line. Moreover, the marked hemolytic potential of this plant is not associated directly to their antiproliferative effect, because seed and stem extracts have not shown a high hemolytic potential. Nevertheless, the cytotoxic activity against PC3 cell line in both cases was evidenced at lower concentrations. S. saponaria L. is a valuable source of metabolites and could therefore have a large relevance on the search of alternative pharmacological therapies for prostate cancer and other applications.














