1. INTRODUCTION
Bioprospecting or biodiversity prospection is defined as the exploration of biodiversity for new resources of social and commercial value (Beattie et al., 2011). Historically, humans have been bioprospecting for all their existence on Earth. Societies have been and still are committed to obtain as many useful things as possible from the nature around. In modern times, the frontiers have been pushed from animals and plants to bacteria, fungus, virus, etc. regarding where the commercially valuable biochemical and genetic resources can come from. This is possible due to the new applications and developments that both demand and enable the discovery of new products. One example is the recent improvements in metagenomics (Madhavan et al., 2017. These efforts can be channeled for better results, such as the case of Colombia, which has been developing with a broad perspective of providing greater benefits for the nation (Malgarejo, 2013). This is an activity of nature exploration, non-destructive, which, through scientific research, aims to obtain useful information derived from the collection of small quantities of biological material for application in medicine, agriculture and industry (Setzer et al., 2003).
Enzymes are biological catalysts and most are of microbial origin (Prabha et al., 2007, Hairchar, 2007). Microbial cellulolytic enzymes are essential for the degradation of cell walls of plants. However, there are limitations for their industrial use, such as the vast diversity of microorganisms in the environment and the complexity to culture them (Gomashe and Bezalwar, 2013). Fungi and bacteria are groups that present extracellular enzymes secreted in the medium in which they are cultured. The mechanism of degradation of cellulose and how microbial communities carry out this work is not yet clear. There are two types of enzymes: extracellular and intracellular. The waste of plant biomass on which they catalyze is composed of lignin, cellulose and hemicellulose (de Gonzalo et al., 2016). Enzymatic hydrolysis of cellulose by microorganisms is a key step in the global carbon cycle, which explains both the fossil fuels deposits and the bioavailability for current living beings. Cellulose degradation by microorganisms is not unique, there being at least five ways to do it with the indispensable presence of cellulases (Wilson, 2011). These microbial cellulases find applications in various industries and constitute a major group of industrial enzymes. One of the biggest industrial interests is bioethanol production, which has experienced considerable impulse in the recent years, due to which many lines of research have been followed (Acharya and Chaudhary, 2012). Some of their recent developments involve metagenomics oriented towards the improvement of processes in biorefineries through identifying new cellulases (Tiwari et al., 2017). One of the agro-industrial products of interest for bioethanol, due to its large amounts of cellulose, is bagasse from sugarcane (Pandey et al., 2000).
The objective of the study was to find microorganisms native to Ecuador, if not better suited or more productive regarding their cellulolytic activity, at least capable of locally and inexpensively produce regular-performing cellulolytic enzymes that can be scaled up to an industrial setting for the degradation of bagasse and perform the analysis of the cost of investment and total production.
2. MATERIALS AND METHODS
2.1. Collection of lignocellulosic material to isolate the strains
The lignocellulosic material was supplied from a panela-producing artisan factory in Balzapamba, from Petroproducción's forest nursery (Amazonia) and from Pedro Vicente Maldonado Station (Antarctica). The samples were washed with distilled water, following the microbiota study by Lauer et al., (2007).
2.2. Qualitative and quantitative screening of cellulolytic enzymes
The isolated sporulating strains were cultured in CMC medium (Carboxymethylcellulose), following the protocol by Shi et al., (2011). For the quantitative assays, an approximate concentration of 5.67 * 109 CFU/g of each purified strain was inoculated into medium specific for endo-, exo-glucanase and filter paper (Lin et al., 2009). In addition, the filter paper activity was determined and calculated according to the methodology established by Adney and Baker (1996) evidenced with dinitrosalicylic acid (Miller, 1959). To evaluate the differences, the 95% confidence intervals for average degradation were established.
2.3. Obtaining the inoculum in a pre-fermenting flask
In the 500 ml pre-fermenting flask, 0.1 g of Bacillus sp. was inoculated with 3.39 g of bagasse, 1 g of CMC and 1.46 g of whey. It was filled with 333.3 ml of drinking water and the medium was sterilized. Local raw material was used.
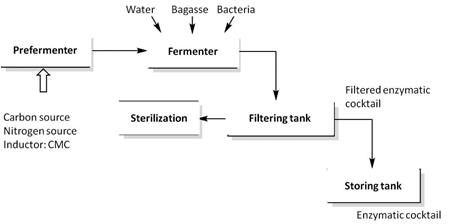
2.4. Obtaining the cocktail in the fermenting flask
In the 1000 ml fermenting flask, the 1:1 ratio of the bacteria produced in the pre-fermenter (10 g) was placed in sugar cane bagasse (10 g) and 110 ml of distilled water. The medium was sterilized. The diagram that shows the scheme for obtaining the enzyme is below (Figure 1):
2.5. Hydrolysis of sugarcane bagasse
The Organosolv protocol employed by Sidiras and Salapa (2015) was used for the sugar cane bagasse. The composition of the material in relation to the percentage of dry matter was: glucan, 49.0%; xylan, 15.6%; lignin, 27.2%. The hydrolysis results published by Salvador et al., (2018) for this strain of Bacillus sp., from Balzapamba were taken into account.
2.6. Economic technical analysis
A production of 50 000 l of ethanol per day was considered. The conditions were the same ones that were maintained in this investigation in the pre-fermenter and fermenter stages. From these data, a scale-up was performed for a production of 2 320 l of bacteria per day, with an enzymatic cocktail production of 1.72 tons of cocktails in 15 hours. The dynamic indicators Net Present Value (NPV), Internal Rate of Return (IRR) and Payback Period (PP) were also calculated.
3. RESULTS AND DISCUSSION
3.1. Isolation of sporulating microorganisms
Hundreds of bacteria and fungi were isolated, being the Balzapamba (Andes) sample the most diverse with a figure of 2.85 CFU. Of these, only those that formed spores were studied (shown in Table 1). The strains with the highest production of cellulases belong to the bacterial species Bacillus sp and the fungus Scopulariopsis sp., according to morphological and biochemical analysis. Literature shows that this fungus belongs to a group that causes fungal tinea.
3.2. Screening of cellulase producing strains
17 sporulating strains producing interesting degradation halos were isolated, as shown in Figure 2. Of these, the fungus produces 6.5 mm of halo, this is the greatest degradation. The bacterial strains Y3 and BAL3 show heterogeneous results due to their variability (Figure 3). Species and cocktails from Antarctica show a low degradation of less than 1 mm.
3.3. Quantitative assays for cellulase production
It was found that the enzymatic cocktails have a higher endoglucanase, exoglucanase and filter paper activity, unlike microorganisms. The results obtained for FPU are: 0.0073 FPU for the Bal 3 bacteria; 0.0041 FPU for the fungus, and 0.0012 FPU for Ant 12. The glucose concentration produced in the endoglucanase assays are: 0.0001 mg/ml for Bal 3; 0.0007 mg/ml for Fungus, and 0.0001 mg/ml for Ant12. The reducing sugar concentration produced in the exoglucanase assays are: 2.7 mg/ml for Bal 3; 3.4 mg/ml for Fungus, and 8.7 mg/ml for Ant12.
The glucose concentration values, obtained from enzymatic cocktails using mathematical models with an R2 of 0.99, for the endobetaglucanase activity are: 2.9 mg/ml, 0.0 mg/ml and 0.8 mg/ml for Fungus, Ant12 and Bal 3, respectively.
In relation to exobetaglucanase activity, the glucose concentration values were 1.0 mg/ml; 5.9 mg/ml and 1.8 mg/ml for fungus, Ant12 and Bal 3. The results obtained for FPU are: 0.013 FPU for the Bal 3 bacteria; 0.0095 FPU for the fungus, and 0.0012 FPU for Ant 12.
3.4. Economic Technical Analysis of Bacillus sp.
For the scale-up, the conditions produced by the Bacillus sp. species (Bal 3) were used. The cost estimates of the investments were made by the methods recommended by (Peter and Timmerhaus, 1991).
3.4.1. Determination of the investment amounts.
Salvador et al., (2018) presented a chart showing the projected equipment expenses increase through the years for industrial purposes. This chart was taken as a reference to calculate the production costs.
Table 2 Calculation of the investment value for the production of the enzymatic cocktail from the bacteria Bacillus sp
| N | Concept | Estimated USD | Amount USD |
|---|---|---|---|
| I | Direct Costs (DC) | A+B+C+D | 453 629 |
| A. Equipment + others | S (1 a 5) | 402 944 | |
| 1. Equipment purchase costs (EC) | 571 776 | 253 424 | |
| 2. Installation including isolation and painting. | 35 % EC | 88 698 | |
| 3. Installation of instruments and control | 6 % EC | 15 205 | |
| 4.Pipes installation | 10 % EC | 25 342 | |
| 5. Electric installations | 8 % EC | 20 274 | |
| B. Buildings | 10 % EC | 25 342 | |
| C. Facilities and services | 10 % CE | 25 342 | |
| D. Land | 0% | 0 | |
| II | Indirect Costs (IC) | A+B+C | 81 176 |
| A. Engineering and supervision | 5 % DC | 22 681 | |
| B. Construction and hiring expenses | 7 % DC | 31 754 | |
| C. Contingency | 5 % IFC | 26 740 | |
| III | Invested Fixed Capital (IFC) | I + II | 534 805 |
| IV | Working Capital | 10 % TWC | 59 423 |
| V | Total Working Capital (TWC) | III + IV | 594 227 |
Table 3 Total Cost of producing bacterial enzymes, taking into account raw materials
| Total Production Cost | Price $/UM | Amount | UM | Cost M$/year |
|---|---|---|---|---|
| 1. Manufacturing Expenses | 444 289 | |||
| Direct costs | 296 789 | |||
| 1.Raw Materials | 175 414 | |||
| Bagasse | 21 | 317515 | Kg/a | 7260 |
| Nutritious broth | 500 | 3062.4 | Kg/a | 165 000 |
| Bacteria | 1654 | 765.6 | L/a | 1654 |
| Water | 0.001 | 1 405 800 | L/a | 1500 |
| 2. Operational labor | 10 %TPC | 85 000 | ||
| 3. Direct supervision 10 % operational labor | 10 % of 2 | 8500 | ||
| 4.Utilities and services | ||||
| Water | 0.001 | 16 000 | L/a | 240 |
| 5.Maintenance and reparation % de IFC | 1058 | |||
| 6. Supplies % of 5 | 52.9 | |||
| 7. Laboratory charges | 8500 | |||
| 8. Patents % CTP | 8500 | |||
| B FIXED EXPENSES | 85 000 | |||
| 1. Depreciation 10 % IFC | 52 910 | |||
| 2. Local fees 1-4% IFC | 52 911 | |||
| 3. Taxes 0,4-1% IFC | 2116.4 | |||
| C. INDIRECT COSTS | 42 500 | |||
| II. General Expenses | 403 289 | |||
| A.Marketing and sales | 17 000 | |||
| B. Management | 17 000 | |||
| C. Research and Development | 17 000 | |||
| D. Financial Interests | 5291.0 | |||
| III. Total Production Cost | 827 578 |
It can be noted in Table 3 that the production cost is $ 827 578 per year. This, divided by 50 000 liters of ethanol per day and by 330 days, gives the cost of enzyme production per liter of ethanol as being $ 0.051. In relation to the commercial enzyme whose cost is USD 0.059 per liter of ethanol (Salvador et al., 2018), the cost reduction is $ 0.0075 per liter of ethanol produced. This cost reduction means a total of $ 123 750 per year, as shown in table 4.
Table 4 Cost reduction in enzymatic expenses when using the enzymatic cocktail
| Enzyme | Cost per liter |
|---|---|
| Cocktail Bacillus sp | $ 0.0515 |
| Commercial enzyme | $ 0.0590 |
| Cost reduction | $ 0.0075 |
In addition, cost reductions are obtained in investment of foreign currency expenditures due to unnecessary purchases, since the necessary supplies are provided in Ecuador for an amount of $ 517 325.12 (Table 5, Table 6). This in turn has a stimulating effect in domestic economy.
Table 5 Investment cost reductions
| Production investment | Cost ($) |
|---|---|
| Total Investment | 587 896 |
| Expenses | 70 571 |
| Expenses reduction | 517 325 |
There is a cost reduction in raw materials since locally produced supplies are used for an amount reflected in Table 6.
Table 6 Raw materials cost reduction
| Raw material | Cost ($) |
|---|---|
| Bagasse | 7 260 |
| Bacteria | 1 654 |
| Water (raw material) | 1 500 |
| Water (general use) | 240 |
| Whey and sugar cane bagasse | 77 220 |
| Total | 87 874 |
In total, a cost reduction in annual imports of $691 649 is achieved, which allows determining investment recovery in relation to expenses abroad (Table 7).
Table 7 Amounts for production investments
| Supplies | Price | Percentage (%) |
|---|---|---|
| Total | $827 578 | 100 |
| Imported | $158 351 | 19.13 |
| National | $669 227 | 80.87 |
What is observed in Table 7 are the cost reductions in imports, since 80,87% of the expenses that will be made for the production of the enzymatic cocktail, use a large number of raw materials locally produced and easily accessible, and the engineering design and manpower will be trained Ecuadorian professionals, encouraging the implementation of new technologies, and innovating production processes. This has a direct impact on the country's technological development.
The following results were obtained: NPV (Net Present Value), $ 198 386.2; IRR (Internal Rate of Return), 19% and PP (Payback Period) of 6 years, Figure 5.
In addition, as shown in Figure 5, the payback period of 6 years starts after the full operation of the production plant of cellulolytic enzymes from Bacillus sp. This time may well serve as a reason to determine that it is not convenient to put it into practice, since the time of money immobilization is maybe too long. A recommendable payback time should be three years or less.
4. CONCLUSIONS
In order to obtain the degradation of lignocellulosic residues in the enzymatic hydrolysis stage, extra cellular enzymatic cocktails are better, unlike the use of microorganisms in the substrate in which the culture medium hydrolysis takes place.
Of the 17 microorganisms analyzed, bacteria of the genus Bacillus was isolated and exhibited activity on crystalline cellulose substrate. This shows that it is feasible to investigate microorganisms that produce a cocktail of autochthonous enzymes.
Biodiversity is a sustainable source that generates value-added products that can provide technological independence.
Through technical economic analysis, it was determined that in the production process of native Bacillus sp. enzymes, cost reductions of ethanol production and investment are achieved, which can have a positive impact on the national economy of Ecuador.