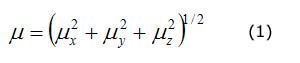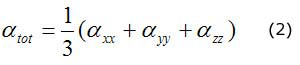Meu SciELO
Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Química
versão On-line ISSN 2224-5421
Rev Cub Quim vol.27 no.2 Santiago de Cuba maio.-ago. 2015
ARTICULOS
Análisis de la estructura y propiedad de óptica no lineal de 1,2 difenil-2-(3-toluidina)-1-etanona tiosemicarbazona mediante cálculos ab initio
Structure and non linear optical property analysis of 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone by ab initio calculations
Lic. Jessica Joyce, Dra. C. Magaly Casals-Hung, Dra. C. América García-López, MSc. Yennys Hernández-Molina, MSc. Félix Nápoles-Escutary
Facultad de Ciencias Naturales, Universidad de Oriente, Santiago de Cuba, Cuba, magalycasals@cnt.uo.edu.cu, america@cnt.uo.edu.cu, yhmolina@cnt.uo.edu.cu
RESUMEN
Se examinó teóricamente la geometría molecular, carga atómica neta, densidad electrónica del átomo, las energías de los orbitales de frontera HOMO y LUMO y las frecuencias vibracionales de la 1,2 difenil-2-(3-toluidina)-1-etanona tiosemicarbazona mediante cálculos ab initio, utilizando el nivel de teoría HF/6-31G(d,p). Se reportan los coeficientes de correlación para las distancias de enlace, ángulos de enlace y frecuencias vibracionales. La distribución de carga, teniendo en cuenta los métodos de Mulliken y de orbitales naturales de enlace muestra posibles sitios de coordinación del compuesto cuando el mismo se coordine con iones de metales de transición. El compuesto exhibe actividad de óptica no lineal.
Palabras clave: 1,2 difenil-2-(3-toluidina)-1-etanona tiosemicarbazona, Hartree-Fock, análisis vibracional, HOMO-HUMO, óptica no lineal.
ABSTRACT
The molecular geometry, net atomic charge and atom electron densities, HOMO-LUMO energy and vibrational frequencies of 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone were examined theoretically using ab-initio method at the HF/6-31G(d,p) level. The correlation coefficients are reported for bond lengths, bond angles and vibrational frequencies. T he Mulliken and natural atomic charges of title molecule reveal the coordination sites when it undergoes complexation with transition metal ions. The title compound exhibit good nonlinear optical activity.
Keywords: 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone , Hartree-Fock, vibrational, HOMO-HUMO, nonlinear optical activity.
INTRODUCTION
Thiosemicarbazones are a class of compounds obtained by condensing thiosemicarbazide with suitable aldehydes or ketones and are well known to possess biological and carcinostatic activities [1, 2]. These biological activities include antitumor, antifungal, antibacterial antiviral, anticancer and antimalarial activities. These properties are often related to metal ion coordination. Lipophilicity, which controls the rate of entry into the cell, is modified by coordination. Also, the metal complex can be more active than the free ligand. In addition, the complex can exhibit bioactivities which are not shown by the free ligand.
Due to its critical role in DNA synthesis and proliferation, iron is a potential target for the treatment of cancer. To this end, the cellular antiproliferative effects of a number of iron specific chelators and their complexes have been examined. A class of chelators with pronounced and selective activity against tumour cells are the thiosemicarbazones. The antitumor properties of heterocyclic thiosemicarbazones are partly related to their ability to inhibit the ribonucleoside diphosphate reductase enzyme, which is essential in DNA synthesis [3].
EXPERIMENTAL
Elemental analyses were carried out on a Thermo Finnigan EA1112 Elementary Analyser Flasch EA 1112. FT-IR spectrum was recorded on a Midac M2000 by using KBr discs.
Preparation of 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone [5].
In a 250 mL round bottom flask appropriate 1,2-diphenyl-2-(3-toluidino)-1-ethanone (5 mmol) and thiosemicarbazide (5 mmoL) were taken. To reaction content 1 mL of pure chorhidric acid was added and dissolved. The mixture was refluxed for 6 hour and then cooled, filtered, washed and recrystallized from absolute ethanol. Yield 89 %; yellow powder; Elemental Analysis calculated for C22H22N4S: C: C, 70,56; H, 5,92; N, 14,96; S, 8,56 %. Found: C, 70,96; H, 5,87; N, 14,94; S, 8,52 %.
Computacional details
The quantum chemical calculations have been performed at Hartree-Fock (HF) method with 6-31G(d,p) as basis set calculations using the Gaussian 03 software package [7], utilizing gradient geometry optimization on a Intel Dual Core 2,8 GHz personal computer. Vibrational frequencies were calculated to make sure no imaginary frequency existing for these structures. The assignments of the calculated wavenumbers is aided by the animation option of Gauss View 3.0 graphical interface for Gaussian programs which gives a visual presentation of the shape of the vibrational modes. Due to the neglect of anharmonicity effect at the beginning of calculation, initially the predicted vibrational wavenumbers by HF/6-31G(d,p) are found to be disagreement with experimental wave numbers. In order to improve the calculated values in agreement with the experimental values it is necessary to scale down the calculated harmonic frequencies. Hence, the vibrational frequencies calculated using HF/6-31G (d,p) level are scaled by 0,899 2 [8].
RESULTS AND DISCUSSION
Geometric structure
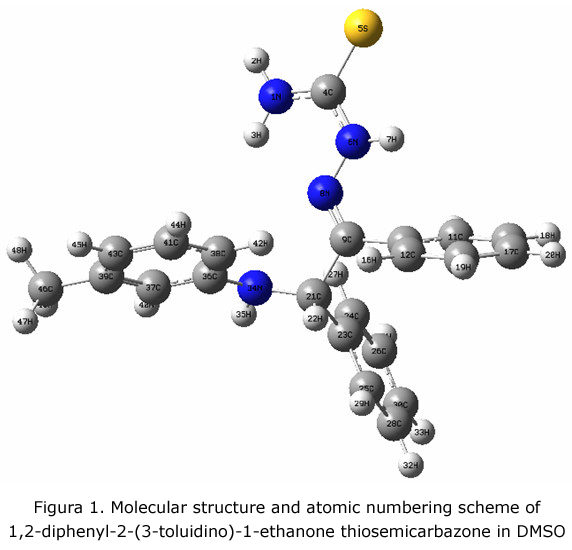
The molecular structure along with numbering of atoms is obtained from Gaussian 03 program (figure 1). At the optimized geometry for the title molecule no imaginary frequency modes were obtained, therefore a true minimum on the potential energy surface was found.
The N8-C9-C21-N34 dihedral angle is a relevant coordinate for conformation flexibility for DTET molecule. Conformation of this molecule is feasible depending on the orientation around C9-C21 bond. The internal rotation potential profile of the C9-C21 bond was obtained by the torsional coordinate N8-C9-C21-N34 to vary in steps of 30° , in gas phase and in solvent DMSO. As it can be seen (figure 2), the internal rotation of C9-C21 bond yielded four minima in approximately 0°, 120°, 210° and 300° in gas phase an two minima in approximately 30° and 270° in DMSO.
The optimized bond lengths, bond angles and dihedral angles of DTET molecule which were calculated by using ab initio method with 6-31G(d,p) basis set in DMSO as solvent are shown in table 1. To the best of our knowledge, crystal data of the DTET molecule are not available in the literature. Therefore, the optimized structure can only be compared with the crystal structure of the other similar systems.
TABLE 1. SELECTED BOND DISTANCE (Å), BOND ANGLES (o) AND DIHEDRAL ANGLES(o)
FOR 1,2-DIPHENYL-2-(3-TOLUIDINO)-1-ETHANONE THIOSEMICARBAZONE
| Parameter | HF/ 6-31G(d,p) | X raya | Parameter | HF/ | X raya |
| Bond distante (Å) |
|
| (continued) |
|
|
| N1-C4 | 1,314 | 1,327 | C46-C39-C43 | 120,152 |
|
| C4-S5 | 1,717 | 1,685 | C46-C39-C37 | 120,671 |
|
| C4-N6 | 1,335 | 1,354 | CC | - | 0,340 |
| N6-N8 | 1,363 | 1,377 | Dihedral angle (o) |
|
|
| N8-C9 | 1,258 | 1,285 | N1-C4-N6-N8 | -2,594 | -7,8 |
| C9-C10 | 1,500 |
| S5-C4-N6-N8 | 177,579 | 174,54 |
| C9-C21 | 1,531 |
| C4-N6-N8-C9 | -179,851 |
|
| C21-N34 | 1,445 | 1,440 5 | N6-N8-C9-C10 | -2,360 |
|
| N34-C36 | 1,389 | 1,381 0 | N6-N8-C9-C21 | 177,971 |
|
| C21-C23 | 1,526 |
| N8-C9-C10-C11 | -69,075 |
|
| C39-C46 | 1,512 |
| N8-C9-C10-C12 | 112,045 |
|
| CC | - | 0,997 1 | N8-C9-C21-N34 | -9,863 |
|
| Bond angle (o) |
|
| N8-C9-C21-C23 | 113,225 |
|
| S5-C4-N6 | 119,585 |
| C9-C10-C11-C13 | -178,918 |
|
| S5-C4-N1 | 122,381 |
| C9-C10-C12-C15 | 179,154 |
|
| N1-C4-N6 | 118,033 |
| C9-C21-C23-C25 | 117,608 |
|
| C4-N6-N8 | 119,413 |
| C9-C21-C23-C24 | -63,258 |
|
| N6-N8-C9 | 119,658 |
| C9-C21-C34-C36 | -84,245 |
|
| N8-C9-C10 | 124,976 |
| C11-C10-C9-C21 | 110,599 |
|
| N8-C9-C21 | 118,509 |
| C12-C10-C9-C21 | -68,281 |
|
| C9-C10-C11 | 120,131 |
| C21-C23-C25-C28 | 179,228 |
|
| C9-C10-C12 | 120,411 |
| C21-C23-C24-C26 | -179,043 |
|
| C10-C9-C21 | 116,514 |
| C21-N34-C36-C38 | 21,012 |
|
| C9-C21-N34 | 113,599 | 108,31 | C21-N34-C36-C37 | -159,698 |
|
| C9-C21-C23 | 106,671 |
| N34-C21-C23-C25 | -117,005 |
|
| C21-C23-C25 | 120,410 |
| N34-C21-C23-C24 | 62,129 |
|
| C21-C23-C24 | 120,475 |
| N34-C36-C37-C39 | -179,440 |
|
| C23-C21-N34 | 109,652 | 112,77 | N34-C36-C38-C41 | 179,425 |
|
| C21-N34-C36 | 113,642 | 122,36 | C46-C39-C37-C36 | -179,569 |
|
| N34-C36-C38 | 123,510 | 122,36 | C46-C39-C43-C41 | 179,639 |
|
| N34-C36-C37 | 118,041 | 119,68 |
|
|
|
CC: correlation coefficient aValues are taken from Refs. [6,9]
Although the correlations coefficientes (CC) for bond lengths and bond angles for DTET are 0,997 1 and 0,340, respectively, these calculated geometrical parameters represent a good aproximation.
Vibrational spectral analysis
The 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone molecule consist of 49 atoms. Its optimized structures exhibits Cs symmetry and consequently all the 141 fundamental vibrations of the molecule are IR activ. The assignments of the calculated wavenumbers is aided by the animation option of Gauss View 3.0 graphical interface for Gaussian program, which gives a visual presentation of the shape of the vibrational modes.
TABLE 2. VIBRATIONAL ASSIGNMENT OF SELECTED FUNDAMENTALS OF
1,2-DIPHENYL-2-(3-TOLUIDINO)-1-ETHANONE THIOSEMICARBAZONE
| Observed fundamentals/cm-1 |
| Assignment |
| VIR | Vscaled/ cm-1 |
|
| 3 133,9 | 3 289,93 | v (N34-H) w |
| 1 500,2 | 1 588,22 | δ (N34-H) s |
| 3 392,8 | 3 456,33 | vas (N1–H) m |
| 3 232,1 | 3 307,36 | vss (N1–H) w |
| 3 321,42 | 3 419,96 | v (N6–H) w |
| 1 660,1 | 1 728,85 | v (C=N8) s |
| 502,3 | 506,57 | v (C–S) m |
| 2 924,1 | 2 889,47 | vas Me, w |
| - | 2 839,47 | vss Me |
| - | 2 820,59 | v (C21–H) |
| 3 062,5 | 3 024,07; 2 937,70 | (C-H) Ph, w |
| 1 602,6 | 1 609,49; 1 579,56 | (C=C) Ph s |
| CC | - | 0,996 5 |
CC: correlation coefficient. v stretching, δ in plane bending, vas asymmetric
stretching, vss symmetric stretching, s strong, m medium, w weak.
Mulliken and natural charge distribution
The calculation of atomic charges play a key role in the application of quantum mechanical calculation to describe the electronic characteristic of molecular system [11]. The parameters like dipole moment, polarizability, reactivity depend on the atomic charges of the molecular systems. The charge distributions over the atoms suggest the formation of donor and acceptor pairs involving the charge transfer in the molecule.
TABLE 3. CHARGE DISTRIBUTION BY THE
MULLIKEN AND NATURAL BOND ORBITAL METHODS OF
1,2-DIPHENYL-2-(3-TOLUIDINO)-1-ETHANONE THIOSEMICARBAZONE
| Atoms | Atomic charges (Mulliken) | Natural charges (NBO) |
| S5 | -0,543 | -0,377 |
| N34 | -0,744 | -0,702 |
| N8 | 0,303 | -0,303 |
| N6 | -0,480 | -0,512 |
| N1 | -0,707 | -0,883 |
NLO properties
Polarizabilities and hyperpolarizabilities characterize the response of a system in an applied electric field. NLO is at in the forefront of current research because of its importance in providing the key functions of frequency shifting, optical modulation, optical switching and optical memory for the emerging technologies in areas such as telecommunications, signal processing, and optical interconnections [12]. NLO techniques are considered as one among the most structure sensitive method to study molecular structures and assemblies. Since the potential of organic materials for NLO devices have been proven NLO properties of many of these compounds have been investigated by both experimental and theoretical methods [13].
The efforts on NLO have been largely devoted to prepare first order NLO materials using theoretical methods and exploring the structure- property relationships. Quantum chemical calculations have been shown to be useful in the description of the relationship between the electronic structure of the system and its NLO response. The computational approach allows the determination of molecular NLO properties as an inexpensive way to design molecules by analyzing their potential before synthesis and to determine the higher order hyperpolarizability tensors of molecule [11].
The calculated polarizability and first order hyperpolarizability values (in a.u.) have been converted into electrostatic units (esu.) (α, 1 a.u. = 0,148,2 · 10-24 esu, ß, 1 a.u. = 8,639 3 · 10-33 esu). The dipole moment, polarizability and first hyperpolarizability are reported in table 4. According to the present calculations, the dipole moment and mean polarizability of 1,2-diphenyl-2-(3-toluidino)-1-ethanone thiosemicarbazone are found to be 10,74 and 17,670 08 · 10 -24 esu. The magnitude of the molecular hyperpolarizability ß , is one of key factors in NLO system. The calculated first static hyperpolarisability ß0 value is equal to 11 309,228 9 · 10-33 esu. The calculated total dipole moment of DTET is approximately three times greater than that of urea and first hyperpolarizability is approximately thirty times to that of urea indicating that the title compound is a good candidate of NLO materials (μ and ß0 of urea are 3,885 1 Debye and 372,8 · 10-33 esu, respectively).
TABLE 4. CALCULATED ELECTRIC DIPOLE MOMENT μ(D),
AVERAGE POLARIZABILITY αTOT(X 10-24 ESU) AND THE FIRST
ORDER HYPERPOLARIZABILITY ß0(X 10-33 -33 ESU)
OF DTET USING USING HF/6-31G(D,P)
| Parameters |
|
| μ | 10,74 |
| αxx | 118,261 |
| αyy | 66,989 |
| αzz | 172,444 |
| αtot | 17,670 0 |
| ßxxx | 43,245 |
| ßyyy | -1 150,746 |
| ßzzz | -622,504 |
| ßo | 11 309,228 9 |
Frontier molecular orbitals (FMOs)
Highest occupied molecular orbitals (HOMOs) and the lowest unoccupied molecular orbitals (LUMOs) are the frontier molecular orbitals (FMOs) which play an important role in the electric and optical properties, as well as in chemical reactions. The HOMO energy characterizes the ability of electron giving. LUMO energy characterizes the ability of electron accepting. The energy gap between HOMO and LUMO characterizes the molecular chemical stability and explain the eventual charge transfer interaction within the molecule, which influences the biological activity of the molecule.
The energy gap represents a critical parameters in determining molecular electrical transport properties because it is a measure of electron conductivity [14]. The increasing value of energy gap in molecule becomes more stable. A molecule with a low energy gap is more polarizable and will exhibit a significant degree of intramolecular charge transfer (ICT) from the electron donor groups to the electron acceptor groups through π conjugated path, indicating a little energy barrier for a possible internal electronic transfer.
The calculated energy value of HOMO of DTET molecule is -7,857 8 eV. LUMO is 2,774 7 eV. The value of energy separation between the HOMO and LUMO is 10,740 eV. This difference in HOMO and LUMO energy supports the charge transfer interaction within the molecule.
CONCLUSIONS
In this paper we have calculated the geometrical parameters and vibrational frequencies and some fundamental vibrations of 1,2 difenil-2-(3-toluidina)-1-etanona tiosemicarbazona molecule by using HF method with 6-31G(d,p) basis set. Scaling factor result is in agreement with experimental. The difference in HOMO and LUMO energy supports the charge transfer interaction within the molecule. The first order hyperpolarizability value confirms molecule has NLO property.
ACKNOWLEDGMENT
We are grateful to VLIR-UOs Program for the financial support to this study.
REFERENCES
1. BERALDO H.; GAMBINO, D., "The Wide Pharmacological Versatility of semicarbazones, Thiosemicarbazones and Their Metal Complexes", Mini-Reviews in Medicinal Chemistry, 2004, 4, 31-39.
2. BLAU, L., et al., "Design, synthesis and biological evaluation of new aryl thiosemicarbazone as antichagasic candidates", European Journal of Medicinal Chemistry, 2013, 67, 142-151.
3. JIANG, Z. G.; LEBOWITZ, M. S; GHANBARI, H. A., "Neuroprotective Activity of 3-Aminopyridine-2-Carboxaldehyde Thiosemicarbazone (PAN-811), a Cancer Therapeutic Agent", CNS Drug Reviews, 2006, 12(1), 77-90.
4. LIU, Z. H.; DUAN C. Y.; HUN. J; YOU, X. Z., "Design, Synthesis, and Crystal Structure of a cis-Configuration N2S2-Coordinated Palladium(II) Complex: Role of the Intra- and Intermolecular Aromatic-Ring Stacking Interaction", Inorganic Chemistry, 1999, 38, 1719-1999.
5. NÁPOLES-ESCUTARY, F. A.; VALE-CAPDEVILLA, R. M.; CASALS-HUNG, M.; LA O-RABIONET, J.; JOYCE, J., "Synthesis and characterization of new α–aminoketone thiosemicarbazones", Revista Cubana de Química, 2012, 24(3), 261-265.
6. MENDOZA-MEROÑO, R.; NÁPOLES-ESCUTARY, F.; MENENDEZ-TABOADA, L.; GARCÍA-GRANDA, S., "1,2-Diphenyl-2-(m-tolylamino) ethanone", Acta Cryst., 2010, E66, o1107.
7. FRISH, A.; NIELSON, A. B.; HOLDER, A. J., GaussView, User manual Gaussian Inc., Pittsburgh, PA, 2001.
8. SCOTT, A. P.; RADOM, L., "Harmonic Vibrational Frequencies: An Evaluation of Hartree-Fock, Møller-Plesset, Quadratic Configuration Interaction, Density Functional Theory, and Semiempirical Scale Factors", J. Phys. Chem., 1996, 100(41), 16502–16513.
9. CASAS, J. S.; GARCÍA TASENDE M. S.; SORDO J., "Main group metal complexes of semicarbazones and thiosemicarbazones. A structural review", Coordination Chemistry Reviews, 2000, 209, 197-261.
10. PRETSCH, E.; BUHLMANN, P.; BADERTSCHER, M., Spektroskopische Daten zur Structuraufklarung organischer Verbindungen, 5.a ed., Berlin/Heidelberg, Springer, 2010.
11. JENSEN, F., Introduction to Computational Chemistry, 2nd ed., Denmark, John Wiley and Sons, 2007.
12. DILLIP, G. R.; REDDY, C. M; RAJU, B. D. P., "Growth and Characterization of Non Linear Optical Material", Journal of Minerals, Materials Characterization and Engineering, 2011, 10(12), 1103-1110.
13. SAJAN, D.; JOE, H. J.; JAYAKUMAR, V. S.; ZALESKI, J., "Structural and electronic contributions to hyperpolarizability in methyl p-hydroxy benzoate", Journal of Molecular Structure, 2006, 785(1-3), 43-53.
14. GECE, G., "The use of quantum chemical methods in corrosion inhibitor studies", Corrosion Science, 2008, 50, 2981-2992.
Recibido: 12/09/2014
Aceptado: 02/12/2014
Dra. C. Magaly Casals-Hung, Facultad de Ciencias Naturales, Universidad de Oriente, Santiago de Cuba, Cuba, magalycasals@cnt.uo.edu.cu