Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Química
versão On-line ISSN 2224-5421
Rev Cub Quim vol.28 no.2 Santiago de Cuba maio.-ago. 2016
ARTICULOS
Obtención de un soporte de afinidad para la purificación de fosfolipasas A2
Obtaining an affinity support for phospholipase A2 purification
MSc. Frenkel Guisado-BourzacI, MSc. Dolores Lázara Romero-Del-SolII, Dr. José Manuel Guisán- SeijasIII, Dr. Jorge González-BacerioII, Dr. Joaquín Díaz BritoII, Dr. Alberto del Monte-MartínezII
IUniversidad de Oriente, Santiago de Cuba, Cuba, fguisado@cnt.uo.edu.cu
IICentro de Estudio de Proteínas (CEP), Facultad de Biología, Universidad de La Habana, Cuba, jogoba@fbio.uh.cu, joaquin43cu@yahoo.es, adelmonte@fbio.uh.cu
IIIInstituto de Catálisis y Petroleoquímica, CSIC, Madrid, España, jmguisan@icp.csic.es
RESUMEN
El objetivo de la presente invención es la síntesis de un soporte de afinidad con un fosfolípido inmovilizado (fofatidilcolina de yema de huevo inmovilizada a un gel amino) como ligando para la purificación de fosfolipasas A2. La Sepharose CL-4B fue activada, oxidada y aminada. La fosfatidilcolina fue inmovilizada covalentemente controlándose mediante la determinación de fosfatos en los materiales de partida y los lavados. La validez de los soportes cromatográficos obtenidos se verificó mediante la purificación de fracciones con actividad fosfolipásica provenientes de las anémonas marinas Condylactis gigantea y Stichodactyla helianthus, así como del veneno de la serpiente Crotalus durisus terrificus. Se obtuvieron perfiles de elución con un máximo típico para este procedimiento y tipo de enzima. La actividad fosfolipásica A2 se verificó cualitativamente a través de una TLC luego de la incubación de los picos obtenidos frente a la fosfatidilcolina purificada y a un sustrato fluorescente: 1-palmitoil–2-NBD-C12-PC.
Palabras clave: cromatografía de afinidad, fosfolipasa A2, inmovilización de fosfatidilcolina de yema de huevo inmovilizada, anémona de mar, gel amino.
ABSTRACT
The synthesis of a glyoxyl-Sepharose support was achieved departing from Sepharose CL-4B firstly activated, oxidized and aminated. Amination process is done in order to obtain an amino gel that could join specific ligands for phospholipases A2 purification. The egg yolk phosphatidylcholine (ePC) was immobilized by covalent method and was controlled by phosphate determination in the departure material and in laundries. This support has the advantage of being able to be chemically modified achieving different affinity supports. The validity of the supports obtained was checked by the addition of chromatography fractions from the sea anemones Condylactis gigantea and Stichodactyla helianthus, and the known snake venom from Crotalus durisus terrificus with phospholipase A2 activity. The typical elution maximum corresponding was obtained. Phospholipase A2 activity was corroborated qualitatively by a TLC-based method after exposure to purified ePC and fluorogenic substrate 1-palmitoil–2-NBD-C12-PC.
Keywords: affinity chromatography, phospholipase A2, egg yolk phosphatidylcholine immobilization, sea anemone, amine gel.
INTRODUCTION
Phospholipases A2 (PLA2) are enzymes that participate in numerous important physiologic and metabolic processes in plants and animals [1-8]. The phospholipase A2 activity is also associated to different pathologies, that's why the purification and further characterization of these enzymes result from big interest, both for basic and applied studies with a leading role in medicine, physiology, nutrition, immunology, microbiology and toxicology [1; 9-14]. Their usefulness is growing in different biotechnological, pharmaceutical and general industries, such as food industry [15, 16] where is not always needed a highly purified enzyme.
Until now, there has been described a diversity of purification procedures for the PLA2, nevertheless, the final yield of many of the proposed routes is low, due to the step numbers in case of complex sources and the natural expression of these enzymes [17-19]. Thus, more powerful procedures such as affinity chromatography are required for the purification of these enzymes.
Affinity chromatography has been a very effective method for the purification of PLA2 [15, 16, 20, 21], therefore the obtainment of supports for this purpose has a great importance and utility when working with these enzymes and the nature of the ligand is essential for a successful affinity procedure, being useful a natural phospholipid for its known affinity [2].
Our research group has achieved the synthesis of a glyoxyl-Sepharose support departing from Sepharose CL-4B firstly oxidized and joined to glycidol, according to Guisán's method [22]. This support has the advantage of being able to be chemically modified achieving different affinity supports. In this work, amination process is done in order to obtain an amino gel that could join specific ligands for PLA2 purification such as presents in Crotalus durisus terrificus snake venoms and Caribbean Sea anemones venoms from Condylactis gigantea and Stychodactyla helianthus.
Theoretical bases
One of the methods of more utility for the purification of proteins is the affinity chromatography [20], hence the importance of obtaining appropriate supports to achieve this end.
For a successfully affinity interaction is essential the nature of the ligand and Leslie [2] proved that PC is a substrate where phospholipases show big affinity.
In the specific case of Condylactis gigantea, it has been proved that soybean PC and egg yolk PC are the best substrates hydrolyzed for this type of enzyme [23], the reason why PC appears to be a ligand that is more adapted for the construction of an affinity support.
In several affinity methods with the variant of a phospholipid ligand or a similar molecule, calcium is needed for the interaction during fixation [24, 25] and elution of fixed proteins is carried out incorporating EDTA, that's why a similar protocol is used herein. This system is essential for the purification of PLA2 and it was demonstrated by Rock and Snider [20], which obtained two enzymes with the above mentioned activity from snake poison with a yield higher than 90 %, while the retention of the enzymes did not happen when buffer fixation did not contained calcium which agrees with the enzyme mechanism proposed for Wells (1972), cited by Rock and Snider [20], and promotes enzyme-substrate interactions which confirms the specific union through affinity across his active center [26 27]. For such a reason, the buffer solution used contains CaCl2 to the ideal concentration for the phospholipase activity of these enzymes in Condylactis gigantea determined by Bárcenas [23].
MATERIALS AND METHODS
Support synthesis for the affinity chromatography.
Obtainment of the amine support
For synthesis, the Sepharose CL-4B support (Sigma-Aldrich, St. Louis, MO, USA) was activated with 1,2-epoxy-3-propanol (glycidol) (Merck & Co., Inc., Whitehouse Station, NJ, USA) and the glycidyl Sepharose CL-4B obtained was then oxidized with NaIO4 (Sigma Chemicals Co) according to the methodology reported by Guisán [22]. Once obtained the glyoxyl-Sepharose CL-4B, the support was aminated according Fernández-Lafuente et al [28]. Thus, the monoamimoethyl-N-aminoethyl-Sepharose CL-4B support (MANA-Sepharose CL-4B) was obtained. Different ethylendiamine concentrations (Merck & Co., Inc., Whitehouse Station, NJ, USA) were used with the objective of achieving different amination degrees for the support.
Phosphatidylcholine oxidation
Two native ligands for chromatographic supports immobilization were assayed, the phosphatidylcholine (PC) from commercial soybean (Merck & Co., Inc., Whitehouse Station, NJ, USA) and from egg yolk purified according to the protocol of Singleton et al [29]. The phospholipids were oxidized, previously to the immobilization, according to the protocol reported by Natori et al [30].
For the oxidation step, 1000 mg of PC were dissolved in 100 ml of acetic acid 90 %. Drop by drop, 200 ml of oxidizing solution (KMNO4 24 mM, NaIO4 20 mM, both Fluka) was added with magnetic stirring during 30 minutes at room temperature. Then, 15 ml of NaHSO4 20 % (Sigma Chemicals Co) are added and lipids extraction is carried out with 750 ml of chloroform: methanol (2:1) in a separative funnel, collecting the chloroform phase below, which is rewashed 3 times with 250 ml of distilled H2O. The chloroform phase is evaporated and the lipid content is re-dissolved in the chloroform: methanol mixture (2:1). All solvents were analytical grade (Sigma Chemicals Co).
Immobilization of oxidized phosphatidylcholine at MANA-Sepharose CL-4B support
The ligand immobilization was carried out according to the method reported by Natori et al [ 30 ] and oxidized egg yolk phosphatidylcholine was used in it. In order to compare, a commercial support AH Sepharose 4B (Pharmacia Biotech, Sweden) was submitted to the same procedure.
Firstly, 10 ml of the monoaminoethyl-N-aminoethyl-Sepharose CL- 4B support were washed with 29,2 ml of dioxane: H2O (V:V) and filtered in porous layer up to dryness.
Next, 98,3 µmol of oxidized phosphatidylcholine are dissolved in 56 ml of the mixture dioxane: H2O (V:V) and the pH is fits to 5,3 with NaOH 0,5 M (Sigma Chemicals Co).
Both, oxidized PC and the amino gel are mixed by a spade stirrer and 793,33 mg of 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (Sigma-Aldrich, St. Louis, MO, USA) are added gently. Agitation remains for 24 hours at room temperature, adjusting the pH to 5.
When the reaction time finishes, the content from reaction mixture is filtered in porous layer and successive washes are realized with 100 ml of dioxane, 100 ml of methanol and finally with 200 ml of NaCl 1M (quality reagent).
Immobilization process characterization
Characterization of the immobilization process by ligand immobilized evaluation
The PC immobilized was determined quantifying phosphates for at least 3 replicas by sample after support degradation by the direct method according to Fiske and Subarrow [31], cited and adjusted by Lanio [32], including a control with the oxidized and aminated support without immobilizing PC. The sample concentration is reported in mg/mL of phosphorous.
Affinity chromatography
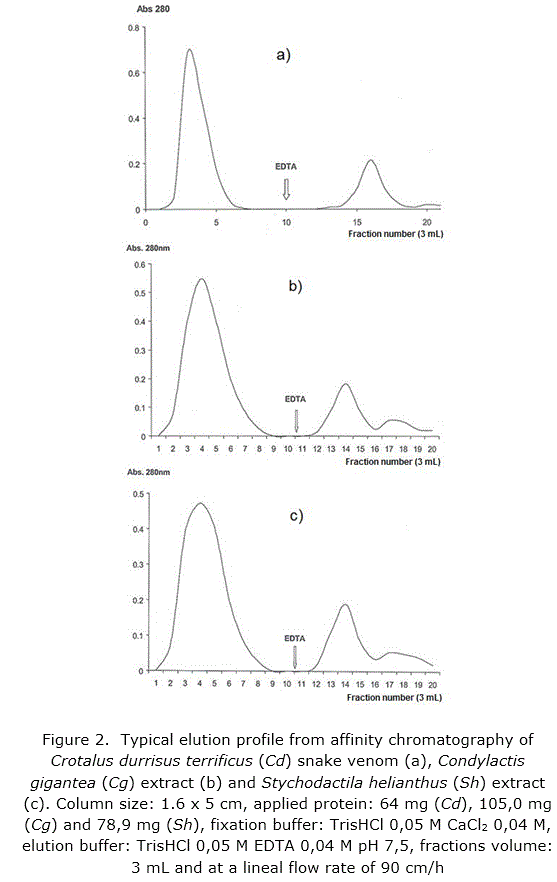
All samples were dialyzed with buffer TrisHCl 0,05 M, CaCl2 0,04 M, pH 7,5 (fixation buffer) before application. Chromatography PC-MANA-Sepharose CL-4B was carried out at 25 °C in a glass column (0,7 x 8 cm). Elution was performed with TrisHCl 0,05 M, EDTA 0,04 M, pH 7,5, at a flow rate of 90 cm/h and fraction of 3mL were collected.
The elution was monitored at 280 nm in an Ultrospec 4000 spectrophotometer (Pharmacia Biotech. Sweden).
Study of the proteins fixation capacity by affinity chromatography in the synthesized support
Proteins fixation capacity for the synthesized support and for commercial AH Sepharose 4 B with oxidized PC ligand was determined by the indirect method. The Condylactis gigantea extract, which contains PLA2 [33], was incubated during 24 hours in semi-batch in a ratio of 0,47 mg protein/g support. After incubation, samples were centrifuged before measuring the protein quantity by Bradford's method [34]. The capacity was calculated subtracting to the total protein applied those who were not fixed.
The maximal adsorption capacity of the support was determined by dynamic methods [35]. Different quantities of proteins from the extract of Condylactis gigantea (10-300 mg) were applied to the column containing the synthesized support. The absorbance of the binding proteins was measured at 280 nm. We considered the maximal saturation where there is no increase in absorbance.
Protein concentration
Protein concentration was determined by the Bradford's method [34] and measured at 280 nm for the protein detection during the chromatographic procedures considering ξ= 1 mg/mL cm1 [ 36 ].
Enzymatic activity
The phospholipase A2 activity was corroborated qualitatively by the method of León et al. [37], a Thin Layer Chromatography (TLC)-based method on a Silica Gel 60 F-254 20x20 plate (Merck, Darmstadt, Germany), using the fluorogenic substrate 1-palmitoil–2-NBD-C12-PC (Avanti Polar Lipids Inc., Alabaster, AL, USA). The presence of the labeled fatty acid in the TLC corroborates this enzymatic activity [38].
Polyacrylamide gel electrophoresis
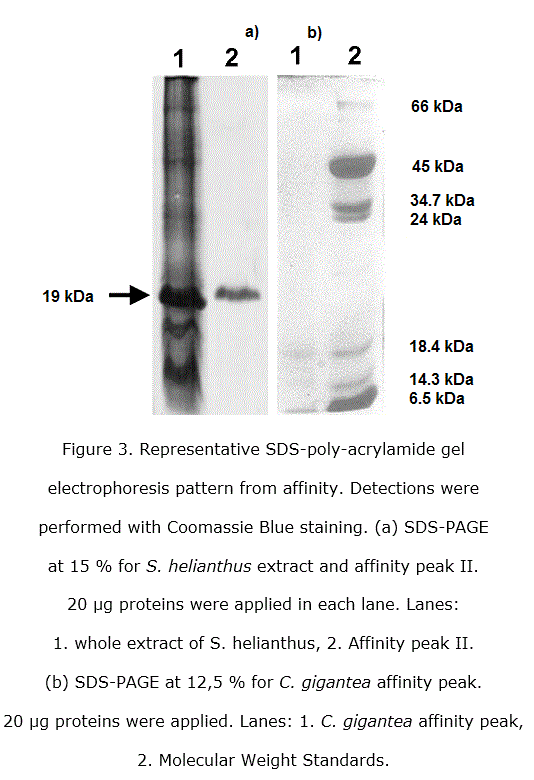
Polyacrylamide gel electrophoresis was carried out according to Laemmli [39], in a vertical chamber with a 12,5% concentration gel for Condylactis gigantea and 15 % for Stichodactyla helianthus. 20 µL of samples previously concentrated and desalinated were applied, using blue bromephenol as indicator and free of 2-mercaptoethanol. As molecular weight standards were used BSA (66000 Da), Ovalbumin (45000 Da), Pepsin (34700 Da), Trypsinogen (24000 Da), ß-lactoalbumin (18400 Da), Lysozyme (14300 Da) and Aprotinin (6500 Da) purchased from Sigma Chem. Co.
Electrophoretic running parameters were: 100 V, 50 mA y 12 watt for 30 minutes during samples concentration in concentrator gel, and a current of 150 V, 100 mA and 12 watt for 1:07 h was applied for separator gel by a power supply Pharmacia LKB- Multidrive XL.
Tincture was effectuated with Coomasie blue R-250 at 0,25 % in methanol 25 %, acetic acid 10 % and water (V/V/V) during 20 minutes. Colorant excess was eliminated by washes with the methanol-acetic acid- water in proportions as previously described during 24 hours and gels were kept into this solution for preserving.
Statistical analysis
STATGRAPHICS CENTURION XV was used for media and standard deviation comparison (Fisher's Least Significant Difference) in all measurements (at least 3 replicas for each tabulated point) for both gels.
RESULTS AND DISCUSSION
The maximum oxidation degree achieved is 50 µmoles aldehyde /mL support. During the amination process different degrees of amino groups in gels were obtained and it was selected the most similar aminated support to those of commercial AH-Sepharose CL-4B described by the manufacturer (11-17 µmol NH2/mL support) for later characterizations.
The analysis of the PC oxidation products was realized by TLC comparing them with a known oxidized PC. Lipid products presence was revealed in a chamber with iodine. Several bands demonstrated that oxidized soybean PC by this methodology did not offer the oxidation product needed while the egg yolk PC did.
The soybean PC oxidation, at tested conditions, does not produce similar reaction products of those who are obtained by the egg yolk PC oxidation. It could be explained by the proper nature of these natural phospholipids. The egg yolk PC possesses generally only a double bound in ω-9 position for the fatty acid placed in sn-2, therefore its oxidation produces a single reaction product preserving its hydrophobic nature while soybean PC is typically polyunsaturated inside the same fatty acid chain, even in both positions (sn-1, sn-2) by which several oxidation products could be obtained according to the assay conditions and a big reduction of the length of its chains, turning out the phospholipid in a hydrophilic polycarboxylated derivative, as observed in TLC with organic solvents. So, even soybean PC is verified as the best substrate hydrolyzed for the phospholipases A2 from Condylactis gigantea [23], it is not the best option during the ligand immobilization process.
Oxidized egg yolk PC bound to amine support was determined by phosphorus (P) quantification (direct method) for both affinity supports, newly and 27 months synthetized by the same conditions (already in use). Results appear in table 1.
TABLE 1. IMMOBILIZATION EVALUATING PARAMETERS BY QUANTIFICATION OF OXIDIZED PC BINDING TO THE SUPPORTS
|
| µg P x g gel-1 | µmoles PC x g gel-1 | µmoles PC x mL gel-1 |
| Synthesized support (MANA-Sepharose CL-4B) | 6,83 ± 2,32 | 0,22 ± 0,07 | 0,31 ± 0,010 |
| Commercial support | 8,64 ± 2,07 | 0,28 ± 0,07 | 0,40 ± 0,010 |
The stability of this support was 92,34 ± 7,61 % (table 2) which is not a statistically relevant reduction for an α= 0,05 and the same mechanism justifies the operational stability of the support, since the reduction of the fatty acid sn-2 prevents the access of the catalytic amino acids towards the bound to hydrolyze it.
TABLE 2. LIGAND BINDING STABILITY IN TIME COMPARED IN TWO DIFFERENTS SYNTHESIZED SUPPORT, MEASURED BY FISKE AND SUBBAROW PHOSPHORUS DETERMINATION METHOD
|
| µg P x g gel-1 |
| Affinity support (time 0) | 6,83 ± 0,31 |
| Affinity support (27 months) | 6,31 ± 0,69 |
Due to known enzymatic mechanism, the buffer solution used contains CaCl2 to the ideal concentration for the phospholipase activity of these enzymes in Condylactis gigantea determined by Bárcenas [23] in order to promote enzyme-substrate interactions which confirms the specific union through affinity across his active center [26, 27].
The comparative study of the protein capacity fixation between the AH-Sepharose 4B and MANA-Sepharose CL-4B is shown in table 3. This capacity measured for the indirect method did not differ statistically for both supports.
TABLE 3. COMPARISON ABOUT PROTEIN CAPACITY FIXATION OF COMMERCIAL AND
SYNTHETIZED AFFINITY SUPPORTS BY SEMI-BATCH SYSTEM
|
| Total proteins (mg) | % Yield |
| C. gigantea eluted fraction (Sephadex G-50) | 0,047 ± 0,003 | 100 |
| MANA-Sepharose (Affinity) | 0,015 2 ± 0,002 | 32,38 ± 2,98 |
| AH-Sepharose (Affinity) | 0,015 4 ± 0,002 | 32,86 ± 2,02 |
During affinity chromatography, protein concentration was followed by DO to 280 nm, choosing an arbitrary extinction coefficient at 280 nm of 1 mg/mL, since these proteins are characterized for presenting a high quantity of aromatic residues for molecule ranges between 13-18 [40], which influences greatly the reading of the D.O. 280 nm that is typical of these amino acids, making more sensitive the detection in front of low protein quantities.
The maximum support aptitude to fix protein proceeding from the gel filtration for Condylactis gigantea whole extract was checked in order to minimize protein losses for extract excess during application to the column. The results of the dynamic support capacity [35] are shown in figure 1.
A lineal increment of the fixed protein is observed until 100 mg of the applied extract after which the tendency is to remains constant. For later affinity chromatography runs it was fixed at 105,0 mg.
Functional characteristic of the synthesized support was corroborated through affinity chromatography runs in column of the Crotalus durisus terrificus snake venom, a recognized genus as source of PLA2 /20/, and the sea anemones Stichodactyla helianthus [11] and Condylactis gigantea [33] which offered a typical profile (figure 2a, b and c respectively) when fractions elute after fixation with calcium ion by addition of EDTA to the elution buffer.
Both, Stichodactyla helianthus and Condylactis gigantea, were purified as showed in the SDS-PAGE applied (figure 3).
The presence of the fluorescence fatty acid in the TLC analysis was determined in protein profiles from affinity peaks of Condylactis gigantea, corroborating by first time the presence of sn-stereospecific activity for purified proteins, indicating that all applied extracts contain PLA2 activity.
Three bands were obtained from electrophoresis in polyacrylamide gels of eluted fractions after concentrating big volumes from affinity peaks of Condylactis gigantea, being still two very tenuous. A similar feature is generally observed for secreted PLA2 from mammals, snakes and invertebrates in general [8]. The enzymatic activity phospholipase A2 was determined qualitatively in each purification step being the most intense, as it was of waiting, the last one from affinity. This result corroborates that the obtained proteins after affinity protocol presents PLA2 activity, at least in one of the three eluted components, a major one and two little bands which could be isoforms as discussed in previously papers [33].
Quantitative results are optimal according to those reported in literature where a very low yield is expected [15, 19, 41].
CONCLUSIONS
The synthesized support shows a great stability in the time and allows the PLA2 purification from different sources like those from snake venom Crotalus durrisus terrificus and the sea anemones Stichodactyla helianthus and Condylactis gigantea.
BIBLIOGRAPHIC REFERENCES
1. BAKER, J. E.; FABRICK, J. A.; ZHU, K. Y., "Characterization of Esterases in Malathion-Resistant and Susceptible Strains of the Pteromalid Parasitoid Anisopteromalus Calandrae", Insect Biochem Molec., 1998, 28(12), 1039-1050.
2. LESLIE, C. C., "Properties and Regulation of Cytosolic Phospholipase A2", The Journal of Biological Chemistry, 1997, (272), 16709-16712.
3. LESLIE, C. C.; VOELKER, D. R.; CHANNON, J. Y.; WALL, M. M.; ZELARNEY, P. T., "Properties and Purification of an Arachidonoyl-Hydrolyzing Phospholipase A2 from a Macrophage Cell Line, Raw 264.7", Biochimica et Biophysica Acta (BBA), 1988, 963(3), 476-492.
4. PARTHASARATHY, S.; STEINBRECHER, U. P.; BARNETT, J.; WITZTUM, J. L.; STEINBERG, D., "Essential Role of Phospholipase A2 Activity in Endothelial Cell-Induced Modification of Low Density Lipoprotein", Proceedings of the National Academy of Sciences, 1985, 82(9), 3000-3004.
5. SCHOENBERG, H. M.; MAYER, J. M.; BEGER, H. G., "Phospholipase A2- from Basic Research to Clinical Reality", Chirurg., 1997, 68(11), 1112-1118.
6. TRONO, D.; SOCCIO, M.; LAUS, M. N.; PASTORE, D., "The Existence of Phospholipase A2 Activity in Plant Mitochondria and Its Activation by Hyperosmotic Stress in Durum Wheat (Triticum Durum Desf.)", Plant Science., 2013, 199–200, 91-102.
7. VAN DEN BOSCH, H., "Intracellular Phospholipase A", Biochem. Biophys. Acta., 1980, 604(2), 191-246.
8. VALENTIN, E.; LAMBEAU, G., "What Can Venom Phospholipases a(2) Tell Us About the Functional Diversity of Mammalian Secreted Phospholipases a(2)?", Biochimie., 2000, 82(9), 815-831.
9. HUDSON, C. J.; LIN, A.; HORROBIN, D. F., "Phospholipases in Search of a Genetic Base of Schizophrenia. Prostaglandin Leukot", Essent. Fatty Acids., 1996, 55(1-2), 119-122.
10. BONVENTRE, J. V., "Roles of Phospholipases A2 in Brain Cell and Tissue Injury Associated with Ischemia and Excitotoxicity J", Lipid Mediat. Cell Signal., 1996, 14(1-3), 15-23.
11. CHÁVEZ, M. A.; WONG, L.; GÓMEZ, T., "Identificación de actividad fosfolipásica en una toxina de la anémona Stichodactyla Helianthus", Scientia, 1988, (1), 76-79.
12. ALBERT, D. H.; SNYDER, F., "Biosynthesis of 1-Alkyl-2-Acetyl-Sn-Glycero-3-Phosphocholine (Platelet-Activating Factor) from 1-Alkyl-2-Acyl-Sn-Glycero-3-Phosphocholine by Rat Alveolar Macrophages. Phospholipase A2 and Acetyltransferase Activities During Phagocytosis and Ionophore Stimulation", The Journal of Biological Chemistry, 1983, (258), 97-102.
13. SILVEIRA, L. B. et al., "Isolation and Expression of a Hypotensive and Anti-Platelet Acidic Phospholipase A2 from Bothrops Moojeni Snake Venom", Journal of Pharmaceutical and Biomedical Analysis, 2013, (73), 35-43.
14. QIU, S.; LAI, L., "Antibacterial Properties of Recombinant Human Non-Pancreatic Secretory Phospholipase A2", Biochemical and Biophysical Research Communications, 2013, 441(2), 453-456.
15. MINCHIOTTI, M.; SCALAMBRO, M. B.; VARGAS, L.; CORONEL, C.; MADOERY, R., "Isolation of Phospholipase A2 from Soybean (Glycine Max) Seeds: The Study of Its Enzymatic Properties", Enzyme and Microbial Technology, 2008, 42(5), 389-394.
16. MADOERY, R.; MINCHIOTTI, M., "Cibacron Blue-Eupergit, an Affinity Matrix for Soybean (Glycine Max) Phospholipase A2 Purification", Enzyme and Microbial Technology, 2006, 38(7), 869-872.
17. IIJIMA, N.; NAKAMURA, M.; VENATSU, K.; KAYAMA, M., "Partial Purification and Caracterization of Pla2 from Hepatopancreas of Red Sea Bream", Nippon Suisan Gakkaishi, 1990, 56(8), 1331-1339.
18. VERGER, R.; FERRATO, F.; MANSBACH, C. M.; PIERONI, G., "Novel Intestinal Phospholipase A2: Purification and Some Molecular Characteristics", Biochemistry, 1982, 21(26), 6883-6889.
19. ZHANG, Y.; XU, T.; CHEN, Q.; WANG, B.; LIU, J., "Expression, Purification, and Refolding of Active Human and Mouse Secreted Group Iie Phospholipase A2", Protein Expression and Purification, 2011, 80(1), 68-73.
20. ROCK, C. O.; SNIDER, F., "Rapid Purification of Phospholipase a from Crotalus Adamantheus Venom by Affinity Chromatography", Journal of Biology, 1975, (250), 6564-6566.
21. TEKE, M.; TELEFONCU, A., "Purification of Bovine Pancreatic Phospholipase A2 by an Affinity Ultrafiltration Technique", Separation and Purification Technology, 2008, 63(3), 716-720.
22. GUISÁN, J. M., "Aldehyde- Agarose Gel as Activated Supports for Inmovilization- Stabilization of Enzimes", Enzyme Microb. Technol., 1988, (10), 375-382.
23. BÁRCENAS, J., "Estudio de algunas de las características funcionales de una fosfolipasa a de la anémona marina condylactis gigantea", Research University of Havana, 1990.
24. VERGER, R.; MIERAS, M. C. E.; DE HAAS, G. H., "Action of Phospholipase a at Interfaces", J. Biol. Chem., 1973, (248), 4023-4034.
25. DEL MONTE MARTÍNEZ, A. et al., "Improved Purification and Enzymatic Properties of a Mixture of Sticholysin I and Ii: Isotoxins with Hemolytic and Phospholipase A2 Activities from the Sea Anemone Stichodactyla Helianthus", Protein Expression and Purification, 2014, (95), 57-66.
26. SIX, D. A.; DENNIS, E. A., "The Expanding Superfamily of Phospholipase A2 Enzymes: Classification and Characterization", Biochim. Biophys. Acta, 2000, 1488(1-2), 1-19.
27. BURKE, J. E.; DENNIS, E. A., "Phospholipase A2 Biochemistry", Cardiovasc. Drugs Ther., 2009, (23), 49-59.
28. FERNÁNDEZ LAFUENTE, R.; ROSEL, C. M.; RODRÍGUEZ, V.; SANTANA, C.; SOLER, G.; BASTIDA, A.; GUISÁN, J. M., "Preparation of Activated Supports Containing Low Pk Amino Group. A New Tool for Protein Inmovilization Via the Carboxyl Coupling Method", Enzyme Microb. Technol., 1993, (15), 550-556.
29. SINGLETON, W. S.; GRAY, M. L.; WHITE, J. L., "Chromatografically Homogeneus Lecithin from Egg Phospholipids", The Journal of American Oil Chemistry, 1965, (42), 53-56.
30. NATORI, Y. K.; ARAI, H.; TAMARI NATORI, Y.; NAJONI, S., "Partial Purification and Properties of Phospholipase a from Rat Liver Mitochondria", Biochem., 1983, (93), 631-637.
31. FISKE, C.; SUBARROW, Y., "The Colorimetric Determination of Phosphoronos J.", Biol. Chem., 1925, (66), 375-400.
32. LANIO, M. E.; ÁLVAREZ, C.; PÉREZ, P., Manual De Prácticas De Biomembranas, La Habana: Editorial Pueblo y Educación, 1988.
33. ROMERO, L. et al. , "Enzymatic and Structural Characterization of a Basic Phospholipase a 2 from the Sea Anemone Condylactis Gigantea", Biochimie., 2010, (92), 1063-1071.
34. BRADFORD, M. M., "A Rapid and Sensitive Method for the Quantification of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding", Anal. Biochem., 1976, (86), 248-254.
35. AIBA, S.; HUMPHREY, A. E.; MILLIS, N. F., Biochemical Engineering, New York: Academic Press Inc 1973, 434 p.
36. SCOPE, R., Protein Purification. Principles and Practice, New York Springer Verlag, 1984.
37. LEÓN, O. S.; HENRÍQUEZ, D. R.; DÍAZ BRITO, J., "Algunas características químico fisicas del tritón x-100 y su relación con la fosfolipasa a de la gorgonia Plexaura Homomalla", Boletín de Inf. Científica del I.Q.B.E. (ACC), 1984, 3(2), 24-34.
38. ABE, A.; KELLY, R.; SHAYMAN, J. A., "The Measurement of Lysosomal Phospholipase A2 Activity in Plasma", J. Lipid Res., 2010, (51), 2464-2470.
39. LAEMMLI, U. K., "Cleavage of Structural Proteins During the Assembly of the Head of Bacteriophage T4", Nature, 1970, (227), 680-685.
40. ALAGÓN, A. C.; MOLINER, R. R.; POSSANI, L. D.; FLETCHER, P. L.; CRONAN, J. E.; JULIA, J. I., "Purification and Characterization of the Phospholipase A", Biochem. J., 1980, (185), 695-704.
41. ZHANG, F.; DONG, L.; CAI, M.; SHEN, J.; WANG, Y., "Heterologous Expression of Lipoprotein-Associated Phospholipase A2 in Different Expression Systems", Protein Expression and Purification, 2006, 48(2), 300-306.
Recibido: 10/07/2015
Aceptado: 28/11/2015
MSc. Frenkel Guisado-Bourzac, Universidad de Oriente, Santiago de Cuba, Cuba, fguisado@cnt.uo.edu.cu