Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Química
versão On-line ISSN 2224-5421
Rev Cub Quim vol.28 no.3 Santiago de Cuba dez. 2016
ARTICULOS
Investigación teórica a nivel semiempírico de Nanotubos de carbono como Nanotubos de ensayo
Theoretical Investigation of Carbon Nanotubes as Nano Test-tube
Dr. C. M. Al-Anber
Department of Physics and Mathematics, Fenner Building, room 081, University Of Hull, Cottingham Road, Hull, HU6 7RX, UK, moh@physicist.net
RESUMEN
Se realizaron cálculos cuánticos (MINDO/3) de las propiedades estructurales de nanotubos de carbono (NTCs) y la adsorción de varios radicales de la glicina y el ácido butírico. Los radicales glicina N-centrada y ácido bitírico C1- centrado presentan la mayoria de los complejos estables con NTCs. El diámetro y longitud de los NTCs sobre las energías antienlazantes entre estas dos biomoléculas con los NTCs muestran un decrecimiento cuando el diámetro del NTC aumenta mientras que las energías enlazantes se incrementan con el aumento de la longitud del NTC. Los radicales de glicina N-centrada se unen preferentemente a los extremos de los NTCs mientras que las moléculas del ácido graso prefieren hacerlo en el medio de la superficie interior del NTC.
Palabras clave: Nanotubos, métodos semiempíricos, interacción, biomoléculas.
ABSTRACT
The quantum calculations (MINDO/3) performed on the structural properties of the CNTs upon adsorption of several glycine and butyric acid radicals. The N-centered glycine and the butyric acid (C1-centered) radicals have most stable complexes with CNT.The diameter and length of the CNT on the anti-binding energies between these two biomolecules with the CNTs show a decrease as the CNT diameter increases while the binding energies increase with CNT length increase. The N-centered glycine radicals prefer to bond at the end of the CNTs while the fatty acid molecules prefer that at the middle of the inside surface of the CNT.
Keywords: nanotubes, semi-empirical methods, interaction, biomolecules.
INTRODUCTION
The nano testtubes are one the advantages of the nanotechnology that depend on the characteristics and behaviour of nanomaterials, being limited to nanoscale dimensions (1–100 nm). The quantum nature for the nanostructures is appeared due to their atomic and molecular sizes . The carbon nanotubes (CNTs) as sheets of graphite wrapped in to a cylindrical form showed a new phenomena in the physics after Iijima discovered it [1-3]. Nanotubes have been used in the new biologic and medicine applications due to the ability for the immobilisation of proteins and enzymes [4,5]. As well, Wong et al. have shown that nanotubes are suited for use as probe tips in applications such as biomolecular probes to the carboxyl groups that are present at the open tip ends and to measure the binding force between single protein-ligand pairs [6]. Furthermore, the nanotubes have potential to penetrate into cells offer the potential for using them for delivery of drugs and antibiotic molecules without toxicity effects [7–10].Furthermore, the nanomaterials are used in biosensing, antigen recognition and DNA hybridization due to their unique properties and the vaccine delivery biomedical applications [11], and the nanotubes present big technological advances in bioengineering too [12]. However, few theoretical studies of the interaction mechanism between the nanotubes and biomolecules, such as an attempt was made to explore the feasibility of nanoparticulate adsorbents as a drug delivery tool for the administration of erythropoietin to the small intestine [13] else the theory methods have been used to study the ether-bonded carbon nanotube[14], compared with an expectation of the broad applications of nanotubes, which tried to introduce the description of the nature of these interactions. However, Mavrandonakis et al. have studied the interaction of the amino acid with CNTs [15,16]. Chen et al. have shown that boron nitride nanotubes, which are non cytotoxic, can be functionalized for interaction with proteins and cells [17]. Furthermore, the influences of others factors, such as the diameters and lengths of the CNTs, on the interaction were considered too [18], also the investigating the CNTs as delivery for the anti-cancer drugs [19, 20], likewise Hesabi et al. studied the skin anti-cancer drugs with the CNTs [21]. Also, the fatty acid (butyric acid) interaction with the CNTs was studied [22]. What is more, others have studied constant molecules interaction with the magnesium oxide nanotubes [23] and also with the boron nitride nanotubes (BNN) [24]. Moreover, Nasrin et al. introduced the thermodynamic view of the communication between the Tacrine, which is the drug for Alzheimer's disease, with boron nitride nanotubes [25]. Both of these reports studied this interaction on the outer surface of the nanotubes and paid little or no attention to study this interaction on the inner surface for these nanotubes.
The aim of the present paper is to introduce a model to the ability to consider the carbon nanotubes CNTs as a nanotest tubes, see figure 1, because there are not available dates about this issue yet. Where we try to examine the interaction of the glycine and the butyric acid radicals on the inner surface of the CNTs, which has an armchair type [26]. Then our purpose is to examine this interaction as a function of the CNT's diameters and lengths. Also, we attempt to investigate the effect of changing the positions of these biomolecules-CNT bonds on the interaction energy. Also , we will try to test the ability to link two glycine molecules on the wall of the CNTs surface, so that the first glycine interact on the inner surface of the CNT and the second one on the outer surface.
COMPUTATIONAL DETAILS
In many cases, the results of the experimental methods are unable to describe accurately small systems of complex chemicals. Theoretical calculation methods can be used to bridge gaps in understanding experimental results. Furthermore, the quantum molecular methods can be used to show properties beyond the scope of current crystallographic methods. Where, the molecular quantum modelsallow us to study optical, magnetic, and electronic properties that cannot easily measured experimentally. Also, the molecular quantum provides the interaction energies that are not provided by X-ray and NMR (nuclear magnetic resonance) experiments, so that the theoretical methods can be used to investigate further and to predict the physical and chemical nature of hydrogen bonding interactions. To determinate the structural and electronic properties of CNTs decorated with the glycine and butyric radicals, we used MINDO/3 (Modified Intermediate Neglect of Differential Overlap version 3). MINDO/3 is the earliest of the Dewar methods [27, 28]. MINDO/3 provides more accurate geometries and heats of formation than CNDO or INDO and has been used widely. The limitations of the INDO approximation, on which MINDO/3 is based, frequently lead to problems of accuracy when dealing with molecules containing heteroatoms. MINDO/3 is particularlysuitable for describing carbocations, including non-classical carbocations, and polynitro organic compounds. In most molecular computations, to perform accurate calculations for a nano-sized system, how can do that without ending in prohibitively large computations such as the DFT methods. The dangling bonds at of the tubes ends were saturated by hydrogen atoms. The resolution of MINDO/3, as implemented in the HyperChem Release 7.52 for Windows Molecular Modeling System programme package (http://www.hyper.com/) was employed for the geometry estimations.
RESULTS AND DISCUSSION
At beginning it was important to determine the most stable isomers of the glycine and the butyric acid radicals on the inside wall of CNTs. Among these possible isomers are the ones from which one hydrogen atom is abstracted from either the N atom or the C atom (see figure 2). So here we adopt three isomers of butyric acid and two isomers from the glycine. Figure 3 shows the linking of these isomers on the inside wall of the CNTs (for a constant length equal to 10.0 ±0.025 Å). Note that in each case; we link the radical –CNT bond in the middle of the inside wall of the CNTs.
Figure 4 shows the binding energies (BE) results for these isomers on the inside wall of the CNTs as a function of the CNTs diameters. The binding energy (BE) of the radicals with the CNTs is according to the equation BE = ERadical + CNT – (ERadical + ECNT), where E Radical + CNT is the energy of the complex of the radical with the CNT. However, there is no binding energy (anti-binding) between these biomolecules on the inside wall of CNTs. As the CNTs diameter increases the anti-binding energies decrease, where this behaviour is the same for all these issues, approximately, but there are shifting among these matters . It was found that the C 1 -centered butyric acid and N-centered glycine radicals have lower anti-binding energies compare with the other isomers approximately, and this behaviour becomes semi-constant for all issues after the CNTs diameter equal to 10.22 Å.
The effect of the CNTs diameters reflects the possibility to make control on the ability of these molecules to pass inside the CNTs. We find that reaction with the single tube wall of the CNT, the butyric acid (C1-centered) and glycine (N-centered) radicals show lower anti-binding with CNTs, thus we further study only these two molecules onthe inside wall of the CNTs. The second important factor is the interaction of the butyric acid (C1-centered) and the glycine (N-centered) radicals on the inside walls butwith different lengths of the CNTs evaluated (for constant diameter equal to10.22 Å). Also, here we put the radical–CNT bond in the middle of the inside wall of the CNTs. The CNTs lengths during their synthesis is a dynamic property.
The binding energy of the both radicals(N-centered glycine and C1-centered butyric acid)on the inside wall of the CNTs are fluctuated with the lengths, as shown in figure 5, so these fluctuating is between the binding and anti-binding as the length of the CNTs increase. However, binding energy between the CNTs with these two biomolecules due to the CNTs lengths increase. There is same behavior for these two biomolecules with CNTs lengths. On another side, the glycine shows more probability to interaction with the CNTs than the butyric acid radical. Thus, we may conclude that the binding of these two molecules on the inside wall of the CNTs depends on the lengths of CNTs more than their diameters.
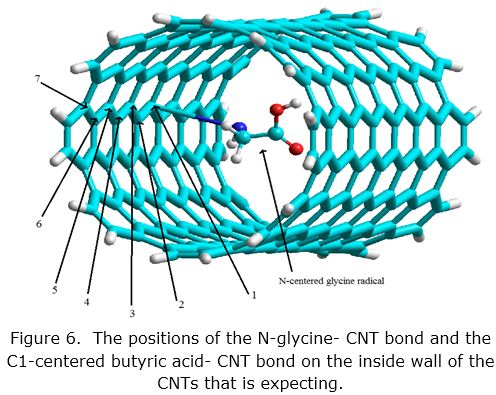
The binding of the N-centered glycine radicals on the inside wall of the CNTs increases as a function of the N glycine –CNT bond position on the internal surface of CNT as this post changes from the middle towards one ofthe CNT two ends from 1,2, 7, as shown in figure 6.
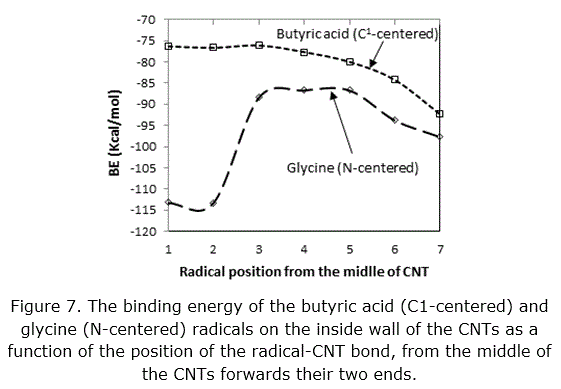
For this purpose the CNTs that adopt with diameter 11.74 Å and length 17.62 Å. We did same for the C1-centered butyric acid radical. The complexes formed by the glycine radical on the inside of the single tube wall are more stable when the reaction occurs at the ends of the CNT, see figure 6. While the binding of the C1-centered butyric acid radicals on inside wall of the CNTs decreases and then increases as a function of the C butyric acid –CNT bond position on the internal surface of CNT, as shown in figure 7.
So we expect there is the probability that the radical glycine interaction with the ends of CNTs and may be will not enter inside the CNTs. The complexes that formed by the C1-centered butyric acid radicals on the inside wall of the CNTs are more stable when the reaction occurs at the middle of the CNT.The butyric acid form complex stable than the Glycine molecule inside the CNTs surface. Due to this point, we expect that the butyric acid radicals will collect in the middle of the cavity of the CNTs and may be will not pass inside the CNT quickly . May be the CNTs can be modified to be like a filter to separate the amino acid and the fatty acid molecules. We tried to investigation the ability of interaction two glycine molecules on the wall of the CNTs surface, so that the first molecule interaction on the inside surface of the CNTs and the second one on the outer surface, see figure 8.
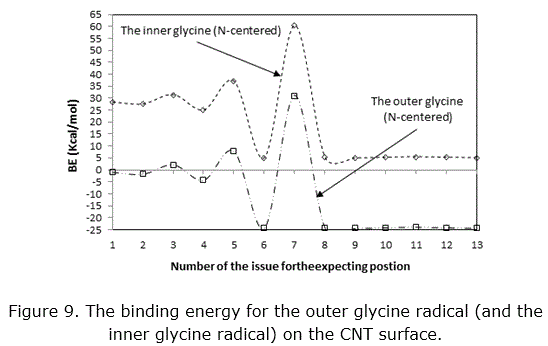
For this purpose the CNTs that adopt here with diameter 11.34 Å and length 12.53 Å. There are many expecting to make linking on this issue, so we start with the same atom on the CNTs as in issue 1. The results in figure 9 show that the outer glycine has binding on the CNT surface while the inner one without binding.
Also, when the two molecules become far from each other, the outer one becomes more binding, while the anti-binding for inner one decreases. Theinner glycine radical will reduce the binding of the outer glycine that on the CNTs surface, so there is fluctuated in this behaviourmay be due to the distances between them. This action becomes constant after position 8, may be attributable to the change the distance between the nitrogen and oxygen atoms of these two glycine molecules. The glycine molecule on the outer surface of the CNTs will decrease the probability to anti-binding the glycine radical on the inside wall of the CNTs, and maybe this issue will not allow the glycine to flow inside the CNTs but without probability to binding on its internal wall.
CONCLUSIONS
In summary, we have performed MINDO/3 calculations on the structural properties of the CNTs upon adsorption of several glycines and butyric acid radicals. Among these isomers, the N-centered glycine and the butyric acid (C1-centered) radicals form stable complexes with CNT compare with the other issues. Our results about the interaction between these two biomolecules with the CNTs are summed up by the following:
1- The results of the diameter and length of the CNT on the anti-binding energies between these two biomolecules with the CNTs show a decrease as the CNT diameter increases while the binding energies increase with CNT length increases.
2- The N-centered glycine radicals prefer to bond at the end of the CNTs while the fatty acid molecules prefer that at the middle of the inside surface of the CNT.
3- The interaction two glycine molecules on the wall of the CNTs surface, one molecule on the inside surface of the CNTs and the second one on the outer surface has an influence on the binding of the both molecules.
4- Maybe the CNTs can be modified to be as a filter to separate the amino acid and the fatty acid molecules.
REFERENCES
1. IIJIMA, S., "Helical microtubules of graphitic carbon", Nature, 1991, 354, 56-58. doi:10.1038/354056a0.
2. IIJIMA, S.; ICHIHASHI, T., "Single-shell carbon nanotubes of 1-nm diameter", Nature, 1993, 363, 603-605. doi:10.1038/363603a0.
3. TSANG, S.; et.al., "A simple chemical method of opening and filling carbon nanotubes", Nature, 1994, 372, 159-162. doi:10.1038/372159a0.
4. DAVISs, J; et.al., "The immobilisation of proteins in carbon nanotubes" Inorganica Chimica Acta, 1998, 272, 261-266. doi:10.1016/S0020-1693(97)05926-4.
5. GUO, Z.; SADLER, P.; TSANG, S., "Immobilization and Visualization of DNA and Proteins on Carbon Nanotubes", Advance Materials, 1998, 10, 701-703.
6. WONG, S.; et.al., "Covalently functionalized nanotubes as nanometer probes for chemistry and biology", Nature, 1998, 394, 52-55. doi: 10.1038/27873.
7. TERRONES, M.; et.al., "Nanotubes: A revolution in materials science and electronics", Topics in Current Chemistry, 1999, 199, 189-234.
8. BALAVOINE, F.; et.al., "Helical Crystallization of Proteins on Carbon Nanotubes: A First Step towards the Development of New Biosensors" Angewandte Chemie International Edition, 38, 1912-1915 (1999).
9. BIANCO, A.; PRATO, M., "Can Carbon Nanotubes be Considered Useful Tools for Biological Applications?", Advance Materials, 2003,15, 1765–1768. doi:10.1002/adma.200301646.
10. PANTAROTTO, D.; et.al., "Synthesis, Structural Characterization, and Immunological Properties of Carbon Nanotubes Functionalized with Peptides", Journal of American Chemical Society, 2003, 125, 6160–6164. doi: 10.1021/ja034342r.
11. PANTHUIS, M., "Vaccine delivery by carbon nanotubes", Chemical Biology, 2003, 10, 897-898. doi:10.1016/j.chembiol.2003.10.005.
12. ZANELLO, L.; ZHAO, B.; HU, H.; HADDON, R. , "Bone cell proliferation on carbon nanotubes", Nano Letters, 2006, 6, 566-567. doi: 10.1021/nl051861e.
13. BASIUK, V., ONIO, M. "Studies of Chemical Reactions on Carbon Nanotube Tips: Effects of the Lower Theoretical Level and Mutual Orientation of the Reactants", Journal of Physical Chemistry B, 2003, 107, 8890-8897. DOI: 10.1021/jp034829m.
14. GUSTAVSSON, S.; ROSEN, A.; BOLTON, K., "Theoretical Analysis of Ether-Group Derivatization at Carbon Nanotube Ends", Nano Letters, 2003, 3, 265-268. DOI: 10.1021/nl025919q.
15. MAVRANDONAKIS, A.; FARANTOS, S.; FROUDAKIS, G., "Glycine Interaction with Carbon Nanotubes: An ab Initio Study", Journal of Physical Chemistry B, 2006, 110, 6048-6050. doi: 10.1021/jp057296l.
16. MAVRANDONAKIS, A.; FROUDAKIS, G.; FARANTOS, C., "Theoretical modeling of glycine radical addition to carbon nanotubes", Reviews on advanced materials science, 2006, 11, 88-91.
17. CHEN, X.; et.al., "Boron Nitride Nanotubes Are Noncytotoxic and Can Be Functionalized for Interaction with Proteins and Cells", Journal of American Chemical Society, 2009, 131, 890–891. doi: 10.1021/ja807334b.
18. Al-ANBAR, M., "Theoretical Semi-empirical Study of the Biomolecules Interaction with Carbon Nanotubes", Journal of Macromolecular Science B, 2011, 50, 2481-2487. doi:10.1080/00222348.2011.557004.
19. Al-ANBAR, M.; ALI, A.; RESAN , S.; Al- MOUALI , A., " The Nitron (Anti-cancer drug) Interaction with Carbon Nanotubes (Delivery): The Semi-Empirical Approach", International Journal of Green Nanotechnology, 2011, 3, 238-243. DOI:10.1080/19430892.2011.628591
20. Al-ANBAR, M; Al-MASOUDI, N., "Theoretical Model of Carbon Nanotubes as Delivery to Fluorouracil (Anticancer)", Revista Colombiana de Química, 2012, 41, 299-310.
21. HESABI, M.; HESABI, M., "The interaction between carbon nanotube and skin anti-cancer drugs: a DFT and NBO approach", Journal of Nanostructure in Chemistry, 2013, 3, 22-28. doi: 10.1186/2193-8865-3-22.
22. Al-ANBAR, M., "Butyric Acid Interaction with Carbon Nanotubes: Modeling by A Semi-empirical Approach", Revista Cubana de Física, 2013, 30, 72-79.
23. Al-ANBAR, M.; Al-LAMI, A.; Al-LAMI, A., "Theoretical Semi-empirical Study of the Amino Acid Interaction with Surface of Magnesium Oxide Nanotubes", European Academic Research, 2014, 2, 1689-1706.
24. RIMOLA, A.; SODUPE, M., "Gas-Phase and Micro-solvated Glycine Interacting with Boron Nitride Nanotubes. A B3LYP-D2* Periodic Study", Inorganics, 2014, 2, 334-350. doi: 10.3390/inorganics2020334.
25. ZAIGHAMI, N.; et. al., "Theoretical study of Interaction between Tacrine and Finite- length Al- doped Carbon and Boron nitride nanotubes: A Semiempirical Drug Delivery Study in Thermodynamic view", Oriental Journal of Chemistry, 2014, 30(4), 1805-1813.
26. TANAKA, K.; YAMABE, T.; FUKUI, K., The Science and Technology of Carbon Nanotubes, First edition, Oxford, Elsevier Science Ltd, 1999. ISBN:0-08-0042696-4.
27. BAIRD, N.; DEWAR, M., "Ground States of s-Bonded Molecules. IV. The MINDO Method and Its Application to Hydrocarbons", Journal of Chemical Physics, 1969, 50, 1262-1274. http://dx.doi.org/10.1063/1.1671186.
28. BINGHAM, C.; DEWAR, S.; LO, H., "Ground states of molecules. XXV. MINDO/3. Improved version of the MINDO semi-empirical SCF-MO method", Journal of American Chemical Society, 1975, 97, 1285-1293. doi: 10.1021/ja00839a001.
Recibido: 7/02/2016
Aceptado: 5/07/2016
Dr. C. M. Al-Anber, Department of Physics and Mathematics, Fenner Building, room 081, University Of Hull, Cottingham Road, Hull, HU6 7RX, UK, moh@physicist.net