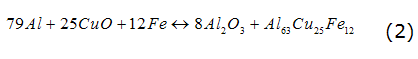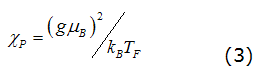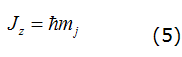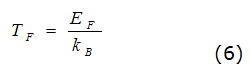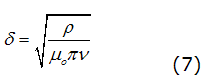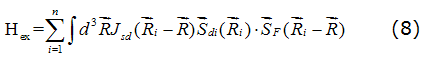My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Cubana de Química
On-line version ISSN 2224-5421
Rev Cub Quim vol.29 no.1 Santiago de Cuba Jan.-Apr. 2017
ARTICULOS
Resonancia Paramagnética Electrónica (RPE) y caracterización de cuasicristal en la fase icosaédrica
Electron Paramagnetic Resonance (EPR) and Characterization of Quasicrystal in the Icosahedral Phase
Dr. Jamshidi, A.L.C.L.I; Dr. Rodbari, R. JII; Dr. Nascimento, L.I; Dr. Barbosa, C.M.B.MI
IDepartment of Chemical Engineering (PPGEQ), Center of Technology and Geosciences (CTG), (UFPE), Brazil, cristina.ufcge@gmail.com
IIDepartment of Materials Science (PPGMTR), Center Sciences of Exact and Natural (CCEN) University Federal of Pernambuco (UFPE), Brazil
RESUMEN
Se estudia la fase icosaédrica de un cuasicristal de Al63Cu25Fe12 producido por fusión en horno de inducción de plasma bajo atmósfera del argón para garantizar la homogeneidad. En las transformaciones de fase, las estructuras formadas por la aleación del cuasicristal con fase icosaédrica ordenada condujeron a una disminución de la conductividad eléctrica lo que conllevó a considerar la presencia de débiles interacciones electrónicas. Para la caracterización microestructural se utilizó Difracción de rayos X, Microscopia Electrónica de Barrido, Espectroscopia de energía dispersiva, Calorimetría diferencial de barrido y Espectroscopia de Resonancia Paramagnética Electrónica (RPE), donde el espectro RPE experimental obtenido arrojó una magnitud de g = 2,036. Los espectros de RPE permitieron visualizar detalladamente los sitios magnéticamente equivalentes y los efectos de la temperatura de transición sobre los espines magnéticos en la muestra ordenada.
Palabras clave: espectro RPE, cuasicristal, caracterización, icosaédrico, paramagnético.
ABSTRACT
This article aims to study the icosahedral phase of a quasicrystal Al63Cu25Fe12 produced by casting in plasma induction furnace under an argon atmosphere to guarantee homogeneity. During phase transformations, the structures formed by the quasicrystalline alloy in ordered icosahedral phase lead to low electrical conductivity, which demonstrates weak electron-electron interactions. The following techniques were used for the microstructural characterization: X-ray Diffraction, Scanning Electron Microscopy, Energy Dispersive Spectroscopy, Thermal Analysis DSC and Spectroscopy Electron Paramagnetic Resonance (EPR). The experimental EPR spectrum obtained presents a resonance g = 2,036. EPR spectra provide a detailed view of magnetic equivalent sites and the transition temperature effects on the magnetic spins in the ordered sample.
Keywords: EPR spectrum, quasicrystal, characterization, icosahedral, paramagnetic.
INTRODUCTION
The quasicrystals are solid atomic structures with periodic and quasi crystallographic symmetry forbidden, since the physical and chemical properties are different from conventional crystalline solids, by presenting a long-range periodicity [1]. The quasicrystalline alloy system AlCuFe and other families of quasicrystal have considerably raised interest because of good physical, chemical, and mechanical properties and tribological: a low electrical and thermal conductivity, good corrosion resistance and oxidation, high hardness and low friction coefficient [2].
Research development on quasicrystal material comes from the thermodynamic stability, non-toxicity property and low production cost of quasicrystalline alloy as well as its great potential in various areas such as health, industry, and technology. Some important applications of this material under study are: photonics, electronic, magnetic, biomaterials and hydrogen storers to be used in catalytic reactions.
The composition range of the quasicrystalline power system Al-Cu-Fe is 58-70 % Al, 20-28 % Fe and 10-14 % Cu, which allow thermodynamic stability in quasicrystalline icosahedral phase. The transformation from the solid phase results from quasicrystalline (ß crystalline, amorphous, icosahedral and intermetallic). In the transition from one phase to another there is presence of amorphous gamma; this process occurs through nucleation from a single crystal of γ-Al2O23 as the core of a layer of amorphous alumina present in quasicrystal under normal temperature and pressure conditions.
The preparation of the alloy Al-Cu-Fe takes place through a thermal aluminum reaction as the equation (1), this overheating is fusion [3]. They are used iron powders, copper and aluminum which are added to reduce the adiabatic combustion temperature and, thereby, avoid vaporization of other components.
Aluminum, copper and iron oxide powders were weighed according to the stoichiometry of equation (2) to produce quasicrystalline materials Al-Cu-Fe with a nominal composition of Al63Cu25Fe12,
The icosahedral phase presents quasicrystalline in the Al-Cu-Fe alloy, which is formed by peritectic solidification of crystalline and amorphous phases of high temperature react with a liquid phase [4].
In view of the formation of quasicrystalline phase from other phases occurred in icosahedral structure, the intermetallic compounds are related to the AlFe AlFeCu [5]. The ß-AlFe structure is a driving force in the formation of quasicrystalline phase AlFeCu. Then, these structures share similar properties and it can be said both are naturally brittle. Therefore, it is expected that the AlFeCu mechanism suffers from being a fragile intermetallic system similar to AlFe [6].
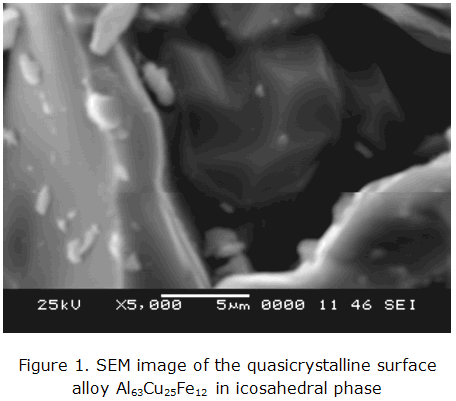
When the quasicrystal is subjected to the eight-hour heat treatment, no decrease in oxidation of the alloy is noticed, which makes possible to obtain and observe the icosahedral structure as shown in figure 1.
Conduction electron is an important study in quasicrystals because a physical understanding of the electronic density of states. Therefore, another interesting aspect that emerges from the location of the unpaired electron spins of the electrons present in quasicrystals in its magnetic moment contained in the transition metals (Mn, Ni, Co, Nb, and Fe) and rare earth metal atoms.
The interaction between the magnetic exchanges unpaired d, f moments leads to magnetic ordering phenomena carrying a magnetic ordering of their spins or antiferromagnetic type at low temperatures [7]. However, to date the quasicrystalline alloy (Al-Mn-Si) and (Al-Mn-B-Pd) exhibited ferromagnetism by which were investigated by electron paramagnetic resonance, verified the existence of ferromagnetic resonance that is reported in ferromagnetic phase. This work aims to study some characterization techniques and magnetic effects by means of electron paramagnetic resonance usage in the icosahedral phase of quasicrystal Al-Cu-Fe.
THEORY
EPR Spectroscopic Study of Al63Cu25Fe12 Quasicrystal in Non-magnetic Phase
The electrical resistivity of quasicrystals presents several orders of magnitude, but it is higher in transition metals. Typical lower resistivity measures at room temperature are in the range ρ ≈ 102 - 103 μΩcm for quasicrystals decagonal and icosahedral with ρ ≈ 102 - 106 μΩcm (the resistivity of aluminum metal ρ(77K) ≈ 0,3 μΩcm) [8]. The resistivity of the quasicrystals and the remaining orders of magnitude lower than that seen in the insulation so that the quasicrystals can be generally considered as weak metals or semimetals.
Paramagnetic Resonance Spectroscopy metal gives rise to two reasons: The first one is the fact that the intensity of the paramagnetic resonance spectroscopy line is proportional to the static spin susceptibility, the conduction electrons, it is of a type Pauli, their order of magnitude can be given by equation (3), [9].
An electron of an atom in the periodic quasi network then has a total angular momentum which is the sum of orbital angular momentum and orbital spin and spin in accordance with equation (4) below [10]:
The relation between the orbital and spin magnetic moments and their angular momentum is different, i.e. that the total magnetic moment, in general, is not parallel to the total angular momentum. Here μB the same relation holds for an atom as a whole, where an electron from an atom then has a total angular momentum. However, the components and μZ maintain a proportional relationship which is expressed as equation (5):
In this case, J is the sum of the angular momentum of all its electrons and the quantum number can assume both integer values as semi-integer, where in unit steps and, is the Fermi temperature of the order of 10 K4 according to equation (6).
This susceptibility remains four orders of magnitude smaller than the type of Curie paramagnetic susceptibility of localized electrons at temperatures of liquid He to a comparable density of electron spins (since electrons are not located in a magnetically ordered state). The second reason for the weakness of the paramagnetic resonance spectroscopy signal is the surface that mediate the effect of conduction electrons.
Spins, where the microwave field penetrates the metal sample only to the radius of the layer of depth order given by equation (7),
The layer effect dramatically reduces the number of excited electrons which contributes to electron paramagnetic resonance signal. In the X-band electron paramagnetic resonance with the resonance frequency of free electrons gF ≈ 2 in v = 9,6 GHZ a typical resistivity ρ ≈ 2.103 μΩcm quasicrystal in a sample which results in a depth of layer δ = 23 μm [11]. For electron paramagnetic resonance up field held in v = 109 GHZ, the depth of the layer reduces the δ = 7 μm. The microwave field can thus excite a small amount of conduction electrons with the surface layer of a single sample. The factor g of conduction electrons in metals is generally not very different from the value of free electrons gF ≈ 2. The reason is that the exchange interaction between the conduction electrons fast moving average is zero and therefore unable to change the resonance frequency. The small difference between actual values and the free electrons is of the order g-gF ≤ 10-2 normally found in metal; it is attributed to the spin-orbit coupling, which causes the electron energy dependent upon the relative orientation between its two angular momentum, orbital and spins [12]. A related situation should be applied to quasicrystals as well. The average conduction electrons should be centered again towards the value of free electrons for the same reason. The spin-orbit coupling, however, deserves more attention in this study.
Electron Paramagnetic Resonance in Quasicrystals Magnetic
Some quasicrystals icosahedral systems such as; Al-Pd-Re, Al-Mn-Pd, Al-Co-Cu and Al-Pd-Ce are perfectly diamagnetic, others may contain paramagnetic centers in addition to itinerant magnetic moments of the conduction electrons. Examples thereof are transition metals containing icosahedral families Al-Pd-Mn, Al-V-Cu, Al-Fe-Ni, Pt-Al-Fe, Al-Co-Ni, Fe-Cr-Al and Cu-Al-Fe, where the Cr, Co, Cu, Ni, Pt, Mn and Fe atoms have unpaired electrons in their energy sublevel "d'' [13]. In this case two types of electronic resonances can be observed in both the conduction electrons in the electron spin location in quasi periodic work. The quasicrystalline alloy containing iron atom Hamiltonian of disturbed crystalline electric field is usually much greater than the spin-orbit coupling should result in small displacements g of localized electron resonances. There is an important difference between the spin-orbit coupling induced displacements of the conduction electrons and localized moments. An electron located is moving in a spherical electrostatic potential with its electrons and the effect of the crystalline electric field meets the electronic orbital angular momentum, whereas it does not affect much the network own potential at the atomic level.
The resulting displacement is therefore very small in quasi periodic quasicrystal work. A conduction electron on the other hand, moves in the crystal a total potential. For regular metals this potential has the periodicity of the structure. In quasicrystals the periodicity of the structure does not exist, so that significant variations in potential sites without translational periodicity. This presents a distribution displacements g which is not necessarily small. The conduction s electrons and localized spins are coupled by an exchange interaction of form of equation (8), [14].
Here Jsd is the exchange coupling constant, SF(Ri-R) is the driving density of free electrons at one point R, Sdi(Ri) it is the total spin of the d electron of the transition metal atom located at point Ri work and the sum is over all atoms of the magnetic structure of quasicrystalline network.This interaction introduces a broadening and a shift of the resonance. However, as the two resonant frequencies are almost equal, the resonance of conduction electrons and localized spins are aligned at the same time have to meet the natural frequencies of the coupled spins. The resonance frequency becomes a function of the frequencies of the two subsystems, the exchange parameter Jsd and the network spin relaxation damping of the two systems. Application of Spin Resonance spectroscopy quasicrystal the sample occurred at the end of a resonant cavity. The quantity measured is the power absorbed by the sample from change when the external field is varied.
In this experiment the reflection was conducted in a band X (irradiation frequency 9.6 GHz, corresponding to the absorption center field 3400 for gF ≈ 2) through a sample powder in quasicrystal Al63Cu25Fe12 icosahedral phase. At room temperature until the liquid He, the spin resonance signal electrons can be detected just below 60 K in a relatively narrow line width, in the range of appeared ΔHpp = (4,5-5)G according to figure 2. Its significance factor g varied in a small range to a low temperature of 4 K mode was observed in the experiment done g= (2,0060-2,0063) whereas the intensity of the line by the reverse enhanced cooling of the Curie temperature 1/T (the Curie law of a paramagnetic material), as disclosed in figure 2b).
The signal obtained left the day of electron paramagnetic resonance experience confirmed the origin of the sample quasicrystal; this was achieved by removed from resonance. But, it is also more likely to originate from impurities that are in the sample, another form of extrinsic quasicrystalline structure.
The quasicrystal in high-field transmission electron spin resonance spectrometer operated in the frequency of microwave 109,270 GHz (corresponding to the absorption center of the field B = 3,890 T to gF = 2). The camp was swept in a range 0-6 T, where we can see that the magnetic field operates at up to 14 T fields. The transmitted signal is detected by a thermal bolometer. In this high-field transmission experiment of electron spin resonance was clearly detected quasicrystal Al63Cu25Fe12, measurements were carried out at a temperature below.
The spectra obtained in this experiment did not appear clear or pure dispersion, but have a slight mixture of absorption. A weak signal with a narrow spectral width is observed near the position of free electrons in B = 3,8684 T, which is obviously the same seen in X band in figure 2.
In figure 3 obtained by electron paramagnetic resonance shows the g factor for the icosahedral phase in quasicrystal Al-Cu-Fe where this shift is because of conduction electrons originating from the iron, because there was a slight variation for a low temperature 4 K, however, the intensity of the line increases by cooling the inverse of the Curie temperature 1/T.
The behavior of the icosahedral phase quasicrystal AlCuFe by some important magnetic properties by electron paramagnetic resonance, and the influence of the magnetic field and the Curie temperature factor1/T, these factors magnetism quasicrystalline the alloy is crucial for applications some industrial sectors.
The structures formed by quasicrystalline alloys have icosahedral phase orderly, provides low electrical conductivity, but is due to consider the location of weak electron-electron interactions. The microstructure effect on quasicrystal is observed by high polarization field, to go analyze heterogeneous spin caused by defects, dislocations and fluctuations of random magneto crystalline.
The Al63Cu25Fe12 quasicrystal has localized magnetic moments on iron atoms (Fe) since only 10-4 Fe atoms have centered moments. The resistivity of the icosahedral quasicrystal Al-Fe-Cu and Al-Pd-Mn are almost the same so that a similar concentration to conduction electrons must exist in both quasicrystals. Furthermore, both quasicrystal families are structurally almost identical.
MATERIALS AND METHODS
The preparation of quasicrystalline alloy consists of an Al62Cu25Fe12 nominal composition as its particle size, having a purity of 99,9 %. The alloy was obtained by melting the pure elements of air. The induction furnace was used with Argon atmosphere controlled at 5.0 with the objective of obtaining a good homogenization of quasicrystalline phase.
We used a high-frequency generator (40 kVA) POLITRON of manufacture. Each element that makes up the quasicrystalline alloy contains 10g; this measurement procedure was performed on a Shimadzu balance. The training method was through solidification in cold hearth furnace to generate a heterogeneous alloy, being a common procedure a mixture of quasicrystalline phase to the crystalline phase. To be a proportional increase of quasicrystalline phase in the sample, it is necessary to heat treatment, so that will favor the peritectic transformation of phases. This was accomplished using the heat treatment in a resistance furnace Nabertherm the mark, where the samples are maintained during the period of 8h and 24 h at a temperature of 1123 K.
The diffraction of X - ray (XRD) was used to monitor the progress of the phases and stability of samples during casting. It was used for both a diffraction SIEMENS D5000 and is used CuK radiation whose wavelength is λ= 1,5406 Å. To examine the morphology of the powders being quasicrystalline using a LEO Scanning Electron Microscope, Model 1430, OXFORD coupled to a probe. The samples after casting and were placed in catalytic tests dispersion in isopropyl alcohol solution. The energy dispersive spectroscopy (EDS) in the sample binds quasicrystalline Al62Cu25Fe12 micrograph was obtained at an energy dispersive analysis after 8 hours heat treatment coupled with the SEM sample which has previously been metallized with gold (average thickness 12nm).
The thermal properties of alloys were analyzed by Differential Scanning Calorimeter (DSC) at 10 °C·min-1 heating rate under N2 flow.
RESULTS AND DISCUSSION
X-ray Diffraction
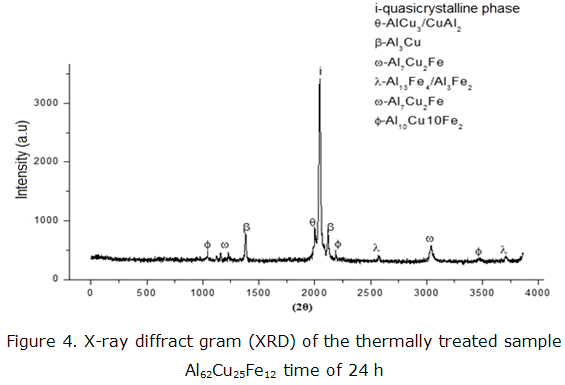
The diffraction spectra of X-rays with the sample Al62Cu25Fe12 stoichiometry is shown in figure 4 respectively for the thermal treatment of 24 hours at 1123 K.
This diffraction peak can identify the phases; the icosahedral quasicrystalline phase (I-phase) and tetragonal phases (θ-AlCu3, θ-CuA12and ß-Al3Cu) and intermetallic (ω-Al7Cu2Fe, λ-Al13Fe4, λ-Al3Fe2, ω-Al7Cu2 Fe and φ-Al10Cu10Fe2). These results also suggest that the ß phase is directly formed from the liquid alloy. Moreover, the ß phase transforms below 873 K to form the λ and θ stages, which are solid solutions induced by the solubility of Cu and Fe.
The intensity of the peaks corresponding to the i-layer is higher than the peaks are specifically related to the ß-phase. However, Al62Cu25Fe12 in the alloy melt, i-phase coexisting with a small amount of AlFe (Cu) solid solution [15]. The composition of Al62Cu25Fe12, have phases in balance with other crystalline phases such as ß-Al2Fe5, γ-Al13Fe4, γ-Al3Fe2, θ-Al2Cu, ω-Al7Cu2Fe andf-Al10Cu10Fe2.
Among these crystalline phases, ω shows a great similarity with the icosahedral Ψ phase. The coordination of Fe atoms is very similar in face-centered cubic structure with Cu being passivity with Al2O3. From the solidification path, the φ phase seems to be formed at equilibrium conditions with high thermodynamic stability, by a peritectic reaction from a crystalline and amorphous intermetallic phase due to phase transitions.
Scanning Electron Microscopy and EDS
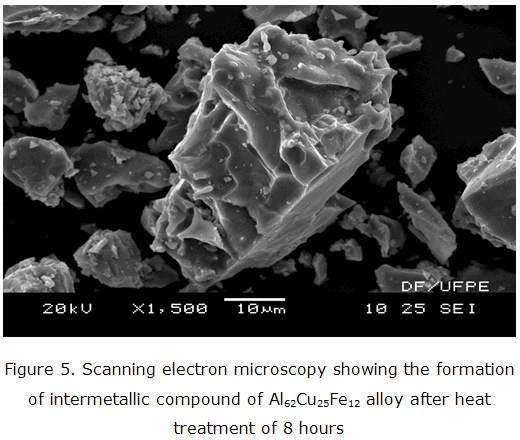
Figures 5 and 6 are respectively represent the results obtained from samples of quasicrystals by Scanning Electron Microscopy and EDS spectrum post after being subjected to heat treatment with 8 hours.
The analysis Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy (EDS) were used for sample quasicrystal and produced figures 5 and 6 with the heat treatment of 8 hours. The image that the Scanning Electron Microscopy (SEM) and Energy Dispersive Spectroscopy spectrum (EDS), view the microstructure of quasicrystalline alloy with surface irregular crystals and geometric uniformity.
The phases present already been processed (ß-crystalline, and intermetallic icosahedral), there are show the strong presence of Fe and Cu conduction electrons which are protected by the thin layer of aluminum oxide which enables the peritectic reaction between phases [16].
In the spectrum of elemental analysis EDS, there is a greater prevalence of aluminum than the other elements (copper and iron) that makes up the quasicrystalline alloy. The existence of γ-Al2O3 favors spinel formation on Al2O4 oxidation in the presence of Cu or CuO. However, the possibility of formation of other oxides such as spine CuFexAl2-xO4, are essential reactions and catalytic composites of Fe3O4 and CuAl2O4 are complex to form a thin film, the passive quasicrystalline structure of the alloy.
Thermal Analysis DSC
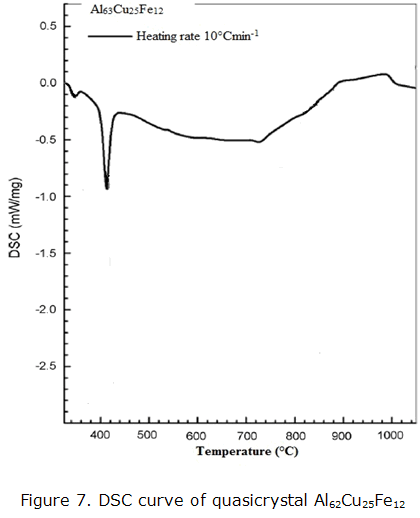
Figure 7 shows the thermal behavior during non-isothermal heating made by Differential Scanning Calorimeter (DSC) conducted at a heating rate of 10° CMIM-1 for quasicrystalline alloy powder during the heating Al62Cu25Fe12 400-1473 K, exothermic events occur are clearly defined and distinguished in a low temperature range of 773 K, however, relates to the formation of AlFe3e-φ ω-Al7Cu2Fe phases, which represents a fusion event or dissolution of the phases (amorphous intermetallic) corresponds to merging with predominance of copper and iron. A more complex sequence of thermal events is observed above 773 K. The increased heating rate causes a moderate change (~293 K) of the phase transition temperatures towards higher values, otherwise the following thermal events does not change. The ω-Al7Cu2 Fe phase is the main precursor of f-phase (i-Al62Cu25Fe12) [17].
As the homogenization of the alloy composition is gradually completed, ω-Al7Cu2 Fe makes if main constituent. The enthalpy of the minimum first peak is smaller than the other two peaks, indicating that the highest concentration of phases of ω-Al7Cu2 Fe typeand (i-Al62Cu25Fe12).This result indicates the need for a detailed analysis of the microstructural evolution and the formation and stability of constituent phases during non-isothermal heat treatments, because this enables rich oxide layer that is essential for catalytic purposes.
CONCLUSIONS
The icosahedral phase becomes stable above 948 K and the crystalline phase a solid solution which controls the formation of the icosahedral phase due to its surface having rotational symmetry that are important to describe the mobility of conduction electrons at the surface.
The nuclear magnetic resonance spectroscopy in quasicrystal is able to detect signs of both itinerant conduction electrons and localized magnetic moments of atoms can provide important information about the electronic properties of quasicrystalline materials.
The use of a conventional X-band in the electron paramagnetic resonance in the conventional apparatus which employs downside scanning a relatively narrow field of 0-1 T.
The sharp widening of the spectrum icosahedral quasicrystal Al63Cu25Fe12 is doubly investigated. The first is the distribution of local electric fields due to the absence of periodicity in quasicrystal reticulum and the second is the exchange interaction energy sublevels in s-d between the conduction electrons and magnetic moments localized d sublevel in Fe.
ACKNOWLEDGEMENTS
The authors acknowledge the North east Center of Strategic Technologies - CETENE and the Department of Physics at UFPE/CCEN that support of this work.
REFERENCES
1. JAMSHIDI, A.L.C.L.; et al . "Use Alloy Quasicrystalline Al 62,2 Cu 25,3 Fe 12,5 for Steam Reforming of Methanol", Journal of Chemical Engineering and Process Technology, 2014, 5(2) 187. doi:10.4172/2157-7048.1000187
2. OLSSON, S.; et al . "Formation of a -approximant and quasicrystalline Al–Cu–Fe thin films", Thin Solid Films, 2012, 526, 74–80.
3. Li, L.; et al. "Large-scale synthesis of Al–Cu–Fe submicron quasicrystals", Scripta Materialia, 2008, 59, 587–590.
4. Srivastava, V.C.; et al. "Bulk synthesis by spray forming of Al–Cu–Fe and Al–Cu–Fe–Sn alloys containing a quasicrystalline phase", Journal of Alloys and Compounds, 2014, 597, 258–268.
5. WIDJAJA, E.J.; MARKS, L. D. "Microstructural evolution in Al–Cu–Fe quasicrystalline thin films", Thin Solid Films, 2003, 441, 63–71.
6. NASCIMENTO, L.; AGOSTINHO, L.C.L.; CAVALCANTI, F.B. "Grouping Model in Fermi Surface Applied to Quasicrystals", Revista Colombiana de Materiales, 2012, (3), 55-62.
7. AZHAZHAA, V.; et al . "The electrical resistivity of Ti-Zr-Ni quasicrystals in the interval 1.3–300 K", Physics Letters, 2003, 319, 539–543.
8. ROY, M. "Formation and magnetic properties of mechanically alloyed Al 65 Cu 20 Fe 15 quasicrystal", Journal of Magnetism and Magnetic Materials, 2006, 302, 52–55.
9. TRSIC, M.; PINTO, M.F.S. Química Quântica: Fundamentos e Aplicações, Editora Manole LTDA., Barueri-SP, 2009.
10. MISRA, S.K.; et al.; "EPR, X-ray, and magnetization studies of metastable alloys Ti 2 Fe, Al 40 Cu 10 Mn 25 Ge 25 and A1 65 Cu 20 Fe 15 as prepared by mechanical alloying", Journal Magnetic Materials, 1995, 150, 430-436.
11. GOMILEKA, J. P. et al. "EXAF study of the Fe local environment in icosahedral AlCuFe and its relation to magnetism of quasicrystals", Solid State Communications, 2012, 123, 527–530.
12. MARION, J.B.; THORNTON, S.T. Classical Dynamics of Particles and Systems. 5a .Ed., Saunders College Publishing, 2004.
13. AGOSTINH O, L.C.L. Estudo da Aplicabilidade dos Quasicristais AlCuFe em Reações Catalíticas na Oxidação do Metanol . Dissertação (Mestrado em Ciências de Materiais). Universidade Federal da Paraíba, João Pessoa-Paraíba, 2009.
14. SAKURAI, J. J. Modern Quantum Mechanics, Revised Edition, Addison-Wesley, 1994.
15. SALES, M.; et al. "Effect of the addition of crystalline ß -phase in Al–Cu-Fe quasicrystalline coatings on their tribological properties", Surface & Coatings Technology, 2007, 201, 6206–6211.
16. SVIRIDOVA, T.A. et al. "Nano quasicrystalline Phase in Mechanically Alloyed and Heat-Treated Al 73 Cu 11 Cr 16", Acta Physica Polonica A. 2014, 126, 99-602.
Recibido: 17/02/2016
Aceptado: 2/09/2016
Jamshidi, A.L.C.L., Department of Chemical Engineering (PPGEQ), Center of Technology and Geosciences (CTG), (UFPE), Brazil, cristina.ufcge@gmail.com