Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Química
versão On-line ISSN 2224-5421
Rev Cub Quim vol.30 no.1 Santiago de Cuba jan.-abr. 2018
ARTICULOS
Formation of cobalt and hydrogen phosphate-based flower-like structures: Influence of polyvinyl alcohol and pyrazole
Formación de estructuras tipo flores basadas en fosfato de cobalto e hidrógeno: Influencia del alcohol polivinílico y el pirazol
Dr. Augusto Iribarren-AlfonsoI, Dra. C. Adaris López-MarzoI, Lic. Ángel Labrada-RodríguezII
IInstituto de Ciencia y Tecnología de Materiales, Universidad de La Habana, La Habana, Cuba, augusto@imre.uh.cu, a_iribarren@yahoo.com
IILaboratorio Central de Criminalística, La Habana, Cuba
ABSTRACT
Phosphate compounds have been object of studies due to their possibilities to be used in catalyst, optoelectronics and optics among other applications. Cobalt and hydrogen phosphate-based radiating sprays of acicular flower-like structures with petals of about 100–300 nm cross-sections were obtained by chemical synthesis. The use of polyvinyl alcohol and pyrazole in the initial dissolutions showed their influences in the variation of size and characteristics of the obtained flower-like structures. In this case the flower petals become more acute, which leads the increasing the specific surface and it facilitates to coat and impregnate the structures with other substance in order to form heterostructures.
Keywords: cobalt phosphate, flower-like structures, chemical synthesis.
RESUMEN
Los compuestos de fosfato han sido objeto de estudio debido a sus posibilidades de ser usados en catálisis, optoelectrónica y óptica entre otras aplicaciones. En el presente trabajo nuevas estructuras tipo flores con forma radial acicular basadas en fosfato de cobalto e hidrógeno con pétalos de alrededor de 100–300 nm se obtuvieron por síntesis química. El uso de alcohol polivinílico y pirazol en la disolución inicial mostraron su influencia en la variación del tamaño y características de las estructuras tipo flores obtenidas. En este caso los pétalos de las flores se hacen más agudos, lo cual lleva al incremento de la superficie específica y facilita cubrir e impregnar las estructuras con otras sustancias para formar heteroestructuras.
Palabras clave: fosfato de cobalto, estructuras tipo flores, síntesis química.
INTRODUCTION
Phosphate compounds have been object of many studies due to their interesting properties and applications [1, 2]. Transition-metal phosphates are of relevant interest for their potential applications in the areas of catalysts, optics, electronics, and magnetic. One of them, cobalt phosphates, has been studied because of their technological interest, which, since its first use as component in semiconductive glasses, already includes applications as ion-selective microelectrodes, catalysts, battery electrodes, glass materials and others [3-6]. Micro and nanostructured crystals and microporous materials have been research interest of several groups because of the need to increase the specific surface of structures [7-9]. Previous works have reported the obtaining of diverse phosphate flower-like geometries structured by plates [10-13]. On the other hand, polyvinyl alcohol (PVA) has been used as surfactant or stabilizer in aqueous dissolutions for the formation of low dimension particles and structures [14, 15]. To our knowledge, pyrazole (HPz) has not been used with that aim, but it could also be useful to modify the structures shape. The presence in the ring of two nitrogen atoms in pyrazole suggests the formation of ionic species that form possible hydrogen and other chemical bonds, which can contribute to the molecular structure of the formed complexes, including their stoichiometry, geometry, and solid-state structures and significant effects on chemical and physical properties [16]. PVA and HPz are highly water soluble and no hazardous hence, they and the solvent do not harm the environment.
The target and achievement of this work were the obtaining of micro and nanodimensioned structures with radiating sprays of acicular flower-like geometry of cobalt and hydrogen phosphates in a film by chemical synthesis using polyvinyl alcohol (PVA) and pyrazole (HPz). The use or not of one or both organic compounds induces different shape flower-like structures. SEM images, EDX, and x-ray diffraction measurements are presented and analyzed.
MATERIALS AND METHODS
Experimental procedure
H3PO4 (85 %), CoCl2·2H2O, and pyrazole (HPz) all analytical grade were purchased from Aldrich, and polyvinyl alcohol (PVA) was PVA 117 (98,5 % hydrolysed) from Chem–Supply Pty Ltd. All reagents were used as received. Dissolutions were prepared as shown in table 1, where pH is also presented. The mixtures were stirred for 15 min in ultrasonic bath and were left in repose for 20 h. For sample preparation a portion of the corresponding dissolution was deposited by drop coating on glass slides and dried by annealing at 70 ° C in air ambient. Semiquantitative pH measurements of the dissolutions were carried out with Merck pH 0-14 indicator paper.
TABLE 1. REAGENT MOLAR RATIO OF THE INITIAL DISSOLUTIONS
| Sample | CoCl2·2H2O | H3PO4 | PVA | HPz | pH |
| H | 1 | 0 | 0 | 6 | 5 |
| 12 | 1 | 0 | 1,5 | 0 | 6 |
| 1-4 | 1 | 1 | 0 | 0 | 1 |
| 2-1 | 0 | 1 | 1,5 | 0 | 1 |
| 3-10 | 0,2 | 1 | 1,5 | 0 | 1 |
| 4-2 | 1 | 1 | 1,5 | 0 | 1 |
| 5-9 | 1 | 1 | 0 | 2 | 2 |
| 6-I | 1 | 1 | 0 | 6 | 3 |
| 8-8 | 1 | 1 | 1,5 | 11 | 3 |
Morphology, size and elemental composition of the structures have been investigated by scanning electron microscopy (SEM) in a Leica 440 microscope equipped for energy-dispersive x-ray spectroscopy (EDX). X-ray diffraction (XRD) characterization of the samples was carried out in a Philips diffractometer working at 45 kV and 40 mA, using Cu Kα radiation.
RESULTS AND DISCUSSION
In order to study the formation of the structures several dissolutions were prepared as shown in table 1. The samples obtained from dissolutions of H3PO4 and CoCl2 lead to the formation of brain-coral-like structures as displayed in the SEM image of figure 1 corresponding to sample 1-4. The structures have diameters of the order of tens micrometers. EDX measurements of the structures reveal presence of Cl, Co, O and P. Chloride was associated with remaining CoCl2 in the structures, which did not react with the phosphoric acid, and then it is possible to subtract from the elemental proportions that corresponding to Co in CoCl2. Thus, rest of Co found in the structures gave an elemental ratio P/Co of 1:1. Under the assumption that they form phosphates, it corresponds to the compound CoHPO4, as corroborated from x-ray diffraction measurements (not shown here).
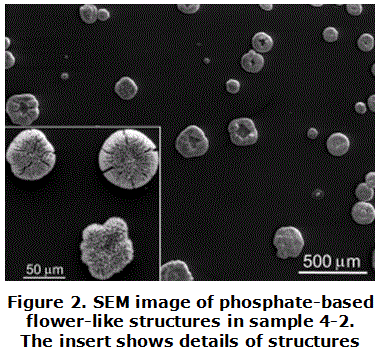
No raising structures were found from the SEM images in sample prepared from dissolution of H3PO4 + PVA (sample 2-1) and HPz + CoCl2 (sample H). However, if some amount of CoCl2 is added to dissolution as in sample 3-10, little structures of about 2 m m diameters were observed by SEM (not shown here). For higher amount of CoCl2 added to the dissolution like in sample 4-2 it is observed the formation of circular rosettes ranged hundreds m m diameter, as shown in figure 2. EDX measurements to the structures gave, after subtracting the amounts corresponding to CoCl2, a relation P/Co with a ratio 1:1, which also would correspond to CoHPO4. The rosettes reach sizes of up to 300 m m, which demonstrates the structure sizes tends to increase due to higher cobalt hydrogen phosphate amounts, together the effect of agglutination of PVA.
From comparing the before-mentioned samples it is possible to affirm that the structures are formed mainly by cobalt hydrogen phosphates with some residual CoCl2. Additionally, it is possible to confirm that the PVA acts as a surfactant agent that confers the dish form to the structures as in sample 4-2, and the present compounds are the same ones in the structures, i. e., CoHPO4 and CoCl2.
The addition of HPz in place of PVA to the H3PO4 + CoCl2 dissolution results in radiating sprays of acicular flower-like structures with independent acute petals, but in this case they only reach diameters up to about 5–10 µm, as exhibited in figure 3. The appearing of these radiating sprays of acicular flower-like structures with lower size and independent petals denoted that HPz influences in it. A feature to takes into account is that from EDX analysis it was fond that the elemental relation P/Co varies according to HPz amount in the dissolution. Thus, the elemental relation P/Co changed from a ratio 3:1 in sample 5-9 to 5:1 in sample 6-I in which the HPz amount is higher. The elemental ratio indicate that a lot of P atoms are associated to hydrogen phosphate, hydrated or not, and the rest is associated to cobalt hydrogen phosphate.
If HPz and PVA are added to the dissolution, i. e., with all the reagents, then the flower-like structures with petals of about 100–300 nm cross-section are also obtained with the same diameter approximately. However, more acute petals can be found as observed in figure 4. The panoramic view of the sample 8-8 in figure 4 exhibits a nearly uniform distribution of structures, which cover about 4 % of the sample surface.
The sample ground was identified as mainly constituted by amorphous PVA and HPz, as corroborated for all the samples where no N and no C were detected by EDX analysis in the structures. However, in some samples the formation of ridge structures can be observed in the ground due to the presence of HPz and CoCl 2 as seen in SEM image. Ridges do not form if HPz is absent; in which case the surface is flatter, as was proved from SEM image of samples H and other images not shown here.
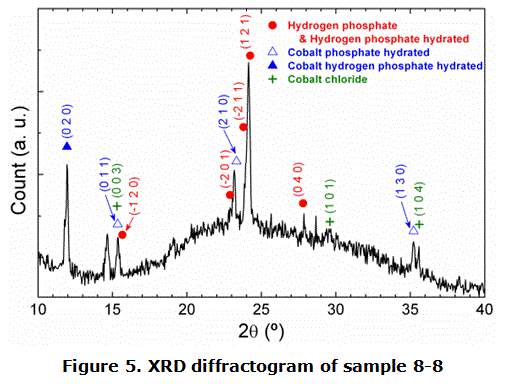
Figure 5 shows the x-ray diffractogram of sample 8-8 and the main peaks are identified [17]. X-ray diffraction measurements determined that sample 8-8 is a mixture of several compounds. The wide band centred at about 2θ≈23º was associated to an amorphous part mainly constituted by PVA and HPz.
The other peaks correspond to orthorhombic cobalt hydrogen phosphate hydrate from dipyramidal class, to monoclinic cobalt phosphate hydrate from prismatic class, to two monoclinic hydrogen phosphates from prismatic class, and to rhombohedral α-CoCl2. These results confirm the presumed compounds from the EDX measurements. Unfortunately, peaks at 2θ≈14,6 º and 2θ≈28,7º kept unidentified. Combining elemental ratios obtained by EDX and DRX diffractogram results it is possible to presume that these unknown peaks correspond to some of the possible cobalt hydrogen polyphosphates. Anyway, it is evident that the flower-like structures are mainly associated to crystalline cobalt phosphates and cobalt hydrogen phosphates with some residual CoCl2, and the shapes and sizes are dependent on the added PVA and HPz.
In general, the acid medium partially prevents the stability of metal pyrazolates and favors the formation of cationic pyrazolium, H2Pz+ [18]. The increase of HPz concentration in dissolution and consequently of pyrazolium reduces the concentration of hydrogen ions in the dissolutions as can be proved from comparing pH in samples 4-2, 5-9, 6-I and 8-8 in table 1. In parallel form the pH increases the higher possibility of Co pyrazolate [19], which also accentuates the pH increase. However, during drying pyrazole segregates due to weakening and breaking of H bonds and it goes to sample ground together PVA. Thus, the concentration of H atoms free from pyrazolium increases and competes with the Co binding to phosphate as demonstrated by comparing elemental relation P/Co from EDX measurements. Those processes with intervention of pyrazole during the drying influence on the shape of the structures, which, besides, is related with the mixture of crystalline hydrogen, cobalt, and cobalt hydrogen phosphates in the flower–like structures.
Cobalt hydrogen phosphate-based flower-like structures with so acute petals have not been previously reported. Further studies on the control of size, compounds, reactions and shape of these structures are being carried out in our research group.
CONCLUSIONS
We obtained cobalt and hydrogen phosphate-based radiating sprays of acicular flower-like structures by chemical synthesis from dissolutions containing H3PO4 and CoCl2 and adding polyvinyl alcohol as surfactant agent and pyrazole as shape modifier. Variations in the concentrations of the dissolution reagent and process conditions lead to changes in the distribution and density as well as the geometry and size of the structures, which are aims of researches in course.
ACKNOWLEGMENTS
One of the authors (A.I.) would like to thank Departamento de Física de Materiales, Universidad Complutense de Madrid, Spain, for use of its facilities. This research is under Project PNCB-51-UH-15, Cuba.
REFERENCES
1. ELIAZ, N.; METOKI, N. "Calcium Phosphate Bioceramics: A Review of Their History, Structure, Properties, Coating Technologies and Biomedical Applications". Materials. 2017, 10, 334.
2. BASAVAPOORNIMA, Ch. et al. "Spectroscopic and pump power dependent upconversion studies of Er3+-doped lead phosphate glasses for photonic applications". J. Alloy. Compd. 2017, 699, 959–968.
3. ZHAN, Y. et al. "The development of cobalt phosphate for bifunctional oxygen electrocatalysis in alkaline solution". Electrochim. Acta. 2017, 227, 310–316.
4. UPADHYAY, A. P.; RASTOGI, C. K.; PALA, R. G. S.; SIVAKUMAR, S. "Desorption retarded optically complemented multiple dye-sensitized photoelectrochemical water splitting system with enhanced performance". Int. J. Hydrogen Energ. 2016, 41, 10727–10736.
5. DI, T. et al. "Enhanced photocatalytic H2 production on CdS nanorod using cobalt-phosphate as oxidation cocatalyst". Appl. Surf. Sci. 2016, 389, 775–782.
6. HAN, X.-B. et al. "Polyoxometalate-Based Cobalt–Phosphate Molecular Catalysts for Visible Light-Driven Water Oxidation". J. Am. Chem. Soc. 2014, 136, 5359–5366.
7. YANG, Q. et al. "Effect of cobalt substitution on nanoporous nickel phosphate VSB-5 catalyst for the catalytic reduction of NO by H2". Catalysis Today. 2017, 297, 64-69.
8. LIU, L. et al. "Hydrothermal synthesis and characterization of a zinc–cobalt phosphate microporous material". Mater. Lett. 2005, 59, 1752–1755.
9. LAGASHETTY, A.; HAVANOOR, V.; BASAVARAJA, S.; VENKATARAMAN, A. "Synthesis of MoO3 and its polyvinyl alcohol nanostructured film". Bulletin of Materials Science. 2005, 28, 477-481.
10. HU, X.; LI, R.; ZHAO, S.; XING, Y. "Microwave-assisted preparation of flower-like cobalt phosphate and its application as a new heterogeneous Fenton–like catalyst". Appl. Surf. Sci. 2017, 396, 1393–1402.
11. ZHENG, J. et al. "Electro-deposited calcium phosphate compounds on graphene sheets: Blossoming flowers". Mater. Lett. 2016, 179, 122–125.
12. NELSON, J. B.; DAVIS, A. M.; WELLMAN, D. M. "Synthesis and size control of cobalt phosphate rosettes using surfactant-templated synthesis". Inorg. Chem. 2009, 48, 10857–10858.
13. SHOJAEE, K.; EDRISSI, M.; IZADI, H.; "Synthesis of flower-like cobalt nanostructures: optimization by Taguchi design". J. Nanopart. Res. 2010, 12, 1439-1447.
14. MA, X. D. et al. "Preparation and characterization of polyvinyl alcohol-capped CdSe nanoparticles at room temperature". J. Colloid Interf. Sci. 2002, 252, 77-81.
15. PATIL, R. C.; RADHAKRISHNAN, S. "Conducting polymer based hybrid nano-composites for enhanced corrosion protective coatings". Prog. Org. Coat. 2006, 57, 332-336.
16. PEREZ, J.; RIERA, L. "Pyrazole complexes and supramolecular chemistry". Eur. J. Inorg. Chem. 2009, 2009, 4913–4925.
17. JCPDS-International Centre for Diffraction Data. PCPDFWIN v. 2.4. Charts, No. 830688, 250408, 390702, 390705, 850446, 2003
18. MUNIZ MIRANDA, M.; NETO, N.; SBRANA, G. "Surface enhanced Raman scattering of pyrazole adsorbed on silver colloids". J. Mol. Struct. 1999, 482–483, 207–212.
19. ADAMS, C. J.; KURAWA, M. A.; ORPEN, A. G. "Coordination chemistry in the solid state: synthesis and interconversion of pyrazolium salts, pyrazole complexes and pyrazolate MOFs". Dalton Trans. 2010, 39, 6974-6984.
Recibido: 24/03/2017
Aceptado: 8/07/2017
Dr. Augusto Iribarren-Alfonso, Instituto de Ciencia y Tecnología de Materiales, Universidad de La Habana, La Habana, Cuba, augusto@imre.uh.cu