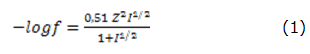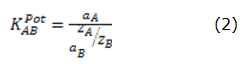Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Química
versión On-line ISSN 2224-5421
Rev Cub Quim vol.30 no.2 Santiago de Cuba may.-ago. 2018
ARTICULOS
A new potentiometric sensor for nitrate using diethylphthalate (DEP) as plasticizer and triocthylmethylammonium chloride (TOMACl) as ionophore
Nuevo sensor potenciométrico para nitrato, usando el dietiloftalato (DEP) como plastificante y el cloruro de trioctilmetilamonio (ClTOMA) como ionóforo
Dra. C. María de los Ángeles Arada-PérezI, Lic. Karel Yasmany Nápoles-FloriánII
IDepartamento de Química. Facultad de Ciencias Naturales y Exactas, Universidad de Oriente, Santiago de Cuba, Cuba, mayarada@uo.edu.cu
IIGeominera Oriente Company. BEU Laboratory, karel.napoles@sclab.minem.cu
ABSTRACT
In this work, a potentiometric sensor for nitrate using diethylphthalate (DEP) as plasticizer, triocthylmethylammonium chloride (TOMACl) as ionophore and poly (vinylchloride) (PVC) as matrix is valued. Some parameters of evaluation of the electrode are presented: slope (S), practical detection limit (PDL), lower limit of linear response (LLLR), lifetime, influence of the pH on the response of the electrode and the potentiometric selectivity coefficients (KABPOT). The constructed electrode exhibited a Nernstian response to NO3- over a wide concentration range (10-6 to 10-2 mol·dm-3), with a slope of -70,71 ± 1,05 mV/dec. It showed a fast response time (20 s). The electrode showed a good performance in the pH range 3 – 9 and lifetime of 2 – 2,5 months. The selectivity coefficients for various ions were calculated.
Keywords: Ion Selective Electrode (ISE), Triocthylmethylammonium Chloride (TOMACl), Diethilphthalate (DEP), Selectivity Coefficient.
RESUMEN
Fue evaluado un sensor potenciométrico a nitrato, empleando el cloruro de trioctilmetilamonio (ClTOMA) como ionóforo y como plastificante el dietiloftalato (DEP) en una matriz de cloruro de polivinilo (PVC). Algunos parámetros de evaluación del electrodo son presentados: pendiente (S), límite práctico de detección (LPD), límite inferior de respuesta lineal (LIRL), tiempo de vida, influencia del pH en la respuesta del electrodo y los coeficientes de selectividad potenciométricos (KABPOT). El electrodo construido mostró una rápida respuesta potenciométrica al ion nitrato en el rango de concentración 10-6 a 10-2 mol·dm-3 con pendiente de -70,71 ± 1,05 mV·déc-1 y un tiempo de respuesta de 20 s. El electrodo mostró una buena respuesta en el rango de pH entre 3 – 9 y tiempo de vida de 2 – 2,5 meses. Los coeficientes de selectividad de varios iones fueron calculados.
Palabras clave: Electrodo de Ion Selectivo (ESI), Cloruro de Trioctilmetilamonio (ClTOMA), Dietiloftalato (DEP), Coeficiente de Selectividad.
INTRODUCTION
The importance of controlling the level environmental pollutants in potable and natural water has generated the interest in the development of new ion selective electrodes (ISEs) for the detection of nitrate [1-3]. Many ionophore have been investigated as sensing agents in electrodes for nitrate ion determination, based on poly (vinylchloride) (PVC) as polymeric membrane, by using salts quaternary ammonium (QAS) [4-7].
Nitrate is an important environmental element. The anion nitrate is rapidly absorbed in the system gastrointestinal itself and it can be reduced to nitrite for effect of present microorganisms in foodstuff of very systems [8]. The nitrite ion, it is when it absorbed time, which rusts iron in the hemoglobin molecule of the ferrous status to the ferric, it is unable to bind reversibly oxygen. The nitrate cause another disease like cancer, defects in the birth, upsets in the central nervous system, etc. For this reason, a variety of methods have been used for determination of ion nitrate such as spectrophotometric [9, 10], chromatography [11], etc., the selective electrodes based on ion exchangers have been reported for nitrate determination [12-15].
Schwake et al. [16] correlate the potentiometric and impedance spectroscopic properties of the membranes with the nature of the plasticizer and the structure of a QAS in a concentration range where the intermediate case of dissociation is realized, membrane selectives do not correspond to the Hofmeister series.
These obtained of QAS structure influences the potentiometric and impedance spectroscopic data for PVC membranes when the QAS molecules are present in the partially dissociated form. The evaluation of bionic potentials showed that an increasing number of carbon atoms in the alkyl chains of the QAS lead to a diminished influence of lipophilic anions. This is explained by the smaller degree of dissociation for the QASs with longer alkyl chain lengths. The decreasing number of cationic sites is the reason for the low selectivity. In contrast, the selectivity towards hydrophilic anions increases with increasing alkyl chain lengths because the association constant increases in this pattern. Membranes with QASs of intermediate lipophilicity show practically no change of bulk resistance with time, a fact that indicates a stable membrane composition.
Kharitonov [17] employed Quaternary ammonium salts containing different functional substituents were tested as ionophore groups in ion exchangers. He calculated the association constants of ionophores in membrane media, solubility products, and partition coefficients in the aqueous solution – membrane solvent system.
Experimental part
MATERIALS AND METHODS
All the reagents used in this study were of analytical grade. Poly (vinylchloride) (PVC) from Fluka as polymeric matrix was used. Triocthylmethylammonium chloride (TOMACl) as ionophore. The plasticizer used was diethylphthalate (DEP) from Merck and Tetrahydrofurane (THF) was analytical grade from Merck. The epoxy conducting resin was prepared by mixing Araldite M and Hardener H form Ciba-Geigy and graphite powder from Merck. The water used in this work was bidistilled water with a conductivity of less than 2 µS/cm-1. The membrane obtained presented a resistance of = 2 k?.
A pH/mv/ o C meter OAKLON digital pH meter with a precision of ± 0,1 mV was used for measuring the potential difference between reference and indicator electrodes. The reference electrode used in this study was a Ag/AgCl HI 5311 double junction electrode and a solution of 0,1 mol·dm-3 of K2SO4 was employed in the external electrode compartment, Microprocessor pH meter 213 Hanna was used for measuring the pH with a combined electrode OAKLON Epoxy boody WD – 35881 – 00 and hot plate y stirner Jenway 1000. LT
Preparation of the membranes
The preparation of the electrode body and the application of the membrane was carried out in a similar manner as the method used for the construction of the all-solid-state ion selective electrodes reported in the literature [19]. The prepared membranes contained 7 % of the ionophore, 64 % of the plasticizer and 29 % of the polymeric matrix (PVC).
Determination of the electromotive force (EMF)
The electromotive force (EMF) determinations were carried out by using a cell to room ambient temperature. The composition of the electrochemical cell was: Ag/AgCl|KCl 0,1 mol·dm-3 |K2SO4 0,1 mol·dm-3 ||Sol.Invest.||memb.PVC|soporte.cond| Cu (s). The calibration curves were used to calculate such parameters as slope (S), practical detection limit (PDL) and lower limit of linear response (LLLR). This was done following the Nernst law through data adjustment by linear regression method. The calibration parameters were obtained by applying the additions method [20], determining the activity of the principal ion by using the Debye-Hückel equation (equation 1):
The effect of the pH response on electrode
The influence of the pH in the response of the PVC membrane electrode was tested using 1,0 x 10-2 mol · dm-3 KNO3 solutions over. The pH was adjusted by using small drops of nitric acid or sodium hydroxide solutions.
The selectivity coefficients
The selectivity coefficients (KABPOT) were determined by using the method of mixed solutions [20] through the equation 2:
All the experimental information was processed using the programs Origin 6.0 [21] and Statgraphics 5.1[22].
The calculation of the confidence interval of the slope, a = 0,05 were determined for a grade of reliability of 95 %, through the equation (3):
RESULTS Y DISCUSSION
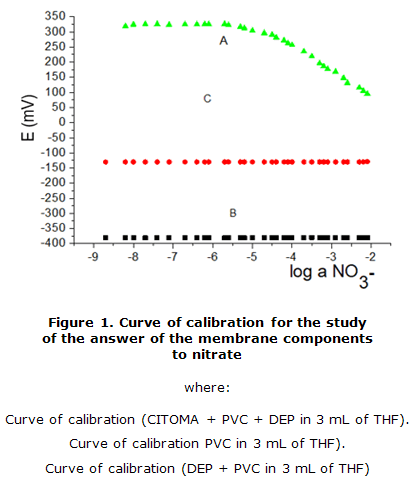
Three blank proofs to corroborate the answer of the membrane came true. As can be seen in figure 1, it could be checked than if membranes did not contain the quaternary salt; the answer of electrodes lacks analytical utility (figure 1).
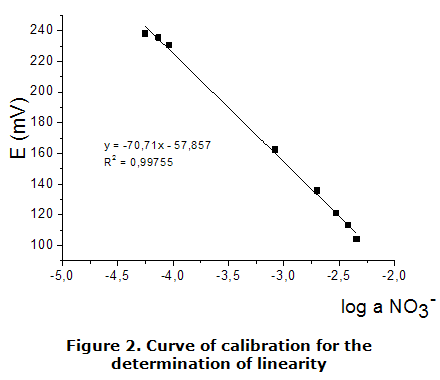
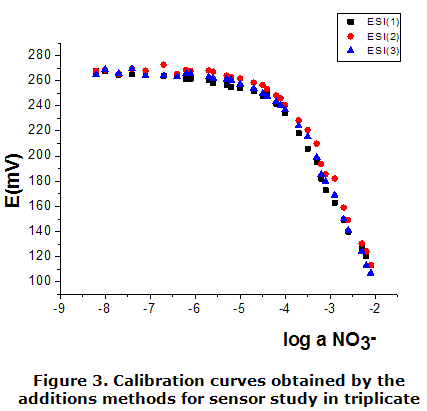
The calibration parameters of the constructed ESIs are shown in table 1. The calibration curves were used to calculate parameters such as: slope (S), practical detection limit (PDL) and lower limit of linear response (LLLR), are shown in figure 2 and figure 3. This was done through data adjustment by linear regression method following the Nernst law.
TABLE 1. CALIBRATION PARAMETERS FOR ELECTRODES OBTAINED BY EMPLOYING THE ADDITION METHOD
| Parameters | DEP (in this works)
|
DOP [18]
|
DBP [18]
|
|
S (mV/dec) | -70,71 ± 1,05 | -57,62 ± 0.91 | -59,22 ± 0,93 |
| R2 | 0,997 55 | 0,999 14 | 0,999 77 |
| PDL (mol·dm-3) | 4,88·10-5 | 2,41·10-5 | 1,90 × 10-5 |
| LLLR (mol·dm-3) | 5,99·10-5 | 5,88 × 10-5 | 3,89 × 10-5 |
| Life time(months) | 2-2,5 | 6,0 | 2,6 |
| Sd (S) | 1,43 | 1,87 | 1,76 |
Sd (S): Standard deviation of the slope
As can be seen from table 1, the value of the over Nernst slope (S) correspond to those expected by Nernst for a monovalent anion, the values obtained for correlation coefficients evidenced the good linearity of the calibration curves. The sensor prepared showed a lower limit of linear response (LLLR) and PDL in order 10-5 mol·dm-3, similar to the obtained with the DOP and DBP [18]. They follow the following order in terms of the solvent mediator employed: DBP (1,90 · 10-5 mol·dm-3) DOP ( 2,41 · 10-5 mol·dm3) DEP ( 4,7·10-5 mol·dm-3). The one for which a dependence as to the PDL and the lipophilicity of the plasticizer used in the membrane cannot be assured sensors.
Analysis of variance (ANOVA) to compare the ESIs's slopes came true. They show the results in the table 2. Previously a contrast to verify if homogeneity among variances existed was accomplished to the comparison. The p Value of Levene's test was 0,922 9 considering that he is superior to the significance level (a = 0,05), statistically significant differences among variances for the 95 % confidence level do not exist.
TABLE 2. ANALYSIS OF VARIANCE ACCOMPLISHED TO THE ESI s
| Source | C.S. | l.g. | M.C. | Quotient -F | Value p |
| Amonggroups | 10,129 5 | 2 | 5,064 74 | 0,03 | 0,973 7 |
| Intra groups | 6 256,05 | 33 | 189,577 |
|
|
C. S.: sumof squares, l.g.: degrees of freedom , M. C.: square means
The p-value is bigger than the significance level ( a = 0,05), which as suggest that statistical differences among the means of three ESIs constructed for a confidence level of the 95 % by which, do not exist they can use indistinctly the ESIs to evaluate his response to nitrate.
Lifetime
The lifetime of the sensors constructed, by using plasticizer DEP, is shown in table 1. As can be seen from table 1, the sensors shown shortest lifetime. When observing the structures of plasticizers in table 1, justifies the reason of why our membrane have the younger time to live, because it has in composition the DEP, since only he has two aliphatic carbon atoms in relation to the DBP and DOP that they have four and eight atoms, respectively; This is what he does that the ionophore exude toward the aqueous phase with bigger facility in relation to the DBP that they have four eight atoms respectively and DOP; This is what he does that the ionophore exude toward the aqueous phase with bigger facility.
Influence of the pH on the response of the electrode
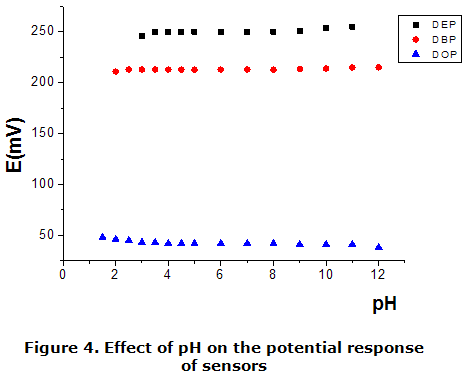
The effect of pH on the response of the electrode was studied over a wide range , by using 1,0 · 10-2 mol · dm-3, by using the correspon ding Reilleys diagrams, are shows in figure 3. An adjustment of pH was performed using dilute sodium hydroxide and sulphuric acid. It is seen from figure 4, that the electrode potential remains constant in pH intervals of 3 to 9,0.
Study of the effect of interfering ions on the selectivity of the constructed ESIs
The potentiometric selectivity coefficients (KABPOT) for a number of mono valence anions were determinated by the mixed solution method, according to IUPAC recommendation. The results are shown in table 3, (interfering 1 · 10-2 mol · dm-3).
TABLE 3. SELECTIVITY COEFFICIENTS (KABPOT) OF VARIOUS INTERFERING ANIONS
| Intf. ions | DEP (in this work) | DBP [18] | DOP [18] |
| Cl- | 1,5 × 10-2 | 1,72 × 10-2 | 6,16 × 10-2 |
| Br- | 3,9 × 10-2 | 1,65 × 10-1 | 2,64 × 10-1 |
| IO3- | 5,61 × 10-2 | 6,77 × 10-2 | 1,48 × 10-2 |
| BrO3- | 5,23 × 10-2 | 5,71 × 10-2 | 1,35 × 10-1 |
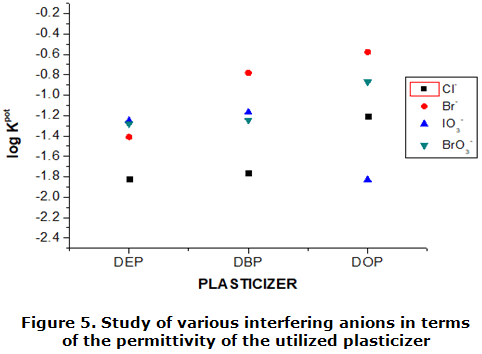
The behavior of the level of interference found in this study in relation to the permitivity of the employed plasticizer can be observed in table 3; as well as the behavior with the same family's another plasticizers using the same ionophore.
As it is observed figure 5, the plasticizer used in this study it has the minor lipophilic character, being the one that presents higher value of dielectric constant (permittivity) (DOP=5,22; DBP=6,58; DEP=7,86) [23]; being the fact that lower interference presents so much for ions chlorides like bromides. It can be observed same as for a same ion his level increases in interference to measure than the plasticizer used not only that lipophilic (bigger carbon chain load). Also, it is noticed that the case of these studied ions stops, the difference in his values of log dies down (KABPOT) to measure than the plasticizer shows bigger value of his dielectric constant.
We can say that examined ions constitute interferences for the determination of the primary ion for which, to be present the same to the hour to examine a sign, it is necessary to take the necessary precautions.
CONCLUSIONS
A new ion selective electrode for nitrate was constructed using TOMACl as ionophore. The nitrate ISEs prepared showed Nernstian slope for nitrate of -70,71 ± 1,05 mV/dec, with a practical detection limit (PDL) of 4,88·10-5 mol · dm-3 and a response time of 20 s. The nitrate ISE developed show a good performance in the pH range of 3 – 9 and had a lifetime of 2 – 2,5 months. The new plasticizer employed DEP with the quaternary ammonium salt TOMACl like ionophore turned out to be the best membrane as to sensibility in relation to the family of plasticizers compared in this research.
REFERENCES
1. LE, G.; BRAVEN, J.; EBDON, L.; SCHOLEFIELD, D. "High-performance nitrate-selective electrodes containing immobilized amino acid betaines as sensors". Anal. Chem. 2002, 74, 2596-2602. ISSN 0003-2700
2. GEORGE, E. et al. "Theoretical and experimental studies of metallated phenanthroline derivatives as carriers for the optimization of the nitrate sensor". Anal. Chim. Acta. 2001, 439, 273–280. ISSN 0003-2670
3. KONG THOO, L P.; ARAUJO, A. N.; MONTENEGRO, M. C. B. S. M.; PEÄ REZ OLMOS, R. "New PVC Nitrate-Selective Electrode: Application to Vegetables and Mineral Waters". J. Agric. Food Chem. 2005, 53(2), 211–215. ISSN 0021-8561
4. PÉREZ, J.; RIOS, A.; FERNÁNDEZ, J. R.; LAPA, R. A. S.; LIMA, J. L. F. C. "Construction and evaluation of ion selective electrodes for nitrate with a summing operational amplifier. Application to tobacco analysis". Talanta. 2001. 53(4) 741–748. ISSN 0039-9140
5. EL DEEN ABBAS, M. N. "Chemically Modified Carbon Paste Electrode for Iodide Determination on the Basis of Cetyl- trimethylammonium Iodide Ion-Pair". Analytical Sciences. 2003, 19(2), 229–233. ISSN 0910-6340
6. OZAWA,S.; MIYAGI, H.; SHIBATA, Y.; OKI, N.; KUNITAKE, T.; KELLER, W. E. "Anion-selective electrodes based on long-chain methyltrialkylammonium salts". Anal Chem. 1996, 68(23), 4149–4152. ISSN 0003-2700
7. HARA, H.; OHKUBO, H.; SAWAI, K. "Nitrate ion-selective coated-wire electrode based on tetraoctadecylammonium nitrate in solid solvents and the effect of additives on its selectivity". Analyst. 1993, 118(5), 549–552. ISSN 0003-2654
8. SAWYER, A.; MC CARTY, C. N. Chemistry for environmental engineering. 3ra Edición. Singapur: Mc Graw–Hill, 1978. ISBN 0070549710, 9780070549715.
9. SANDER, J.; SEIF, F. "Bacterial reduction of nitrate in human stomach as a cause for nitrosamine formation". Arzneim.-Forsch. 1969, 19, 1091–1093. ISSN 2194-9387
10. BELGRANO, R.; COLASURDO, V.; DÍAZ, O. "Métodos ultravioleta selectivo y de reducción con hidracina en la determinación del ion nitrato en aguas subterráneas". Quím. Nova. 2003, 26(5), 766-768. ISSN 1678-7064
11. BOY FERNÁNDEZ, M. E. "Utilización de la cromatografía iónica en el análisis de muestras de agua de drenaje y de solución de suelo". Tesis Doctoral. Universidad de Sevilla, 1992.
12. ARADA PÉREZ, M. de los A.; YAZDANI PEDRAM, M. "Chemical sensor based on tetradecil ammonium nitrate". J. Chil. Chem. Soc. 2013, 58(1), 1415–1418. ISSN 0001-9704
13. ARADA, M. de los A.; YAZDANI, M.; MARIN, J.; SPECK, A. "Estudio del reconocimiento molecular de un portador móvil neutro usado como un electrodo all solid stated a nitrato". Revista Afinidad. 2009, 66(540), 134–138. ISSN 0925-4005
14. ARADA, M. de los A.; YAZDANI, M.; PÉREZ SAAVEDRA, J. J. "Sensores Electroquímicos basados en Sales cuaternarias de amônio". Revista Cubana de Química. 2008, 20(1), 31–38. ISSN 2224-5421
15. RADA PÉREZ, M. de los A.; PÉREZ MARÍN, L.; CALVO QUINTANA, J,; YAZDANI PEDRAM, M. "Influence of different plasticizer on the response of chemical sensors based on polymeric membrane for nitrate ion determination". Sensors and Actuator B Chemical. 2003, 89(3), 262–268. ISSN 0925-4005.
16. SCHWAKE, A. et al. "Potentiometric properties and impedance spectroscopic data of poly(vinyl chloride) membranes containing quaternary ammonium salts of different chemical structure". Anal. Chim. Acta. 1999, 393, 19 – 28. ISSN 0003-2670
17. KHARITONOV, S. V. "Ion selective electrode for the determination of sulfadimezine". J. of Anal. 2001, 56(7), 671–675. ISSN 1588-2780
18. ARADA PÉREZ, M. de los A. et al. "Effect of plasticizer type on the potentiometric selectivity coefficient (KPot) of electrodes for nitrate ion determination constructed by using PVC as polymeric membrane". Revista Cubana de Química. 2003, 15(3), 36–43. ISSN 0258-5995
19. MARTÍNEZ, E. Sensores potenciométricos all-solid-state d´uera. Tesis Doctoral. Universidad Autónoma de Barcelona, 1989.
20. IUPAC. Recommendations for publishing manuscripts on ion selective electrodes. Pure Appl. Chem. 1980, 53(1), 139-143. ISSN 1365-3075
21. ORIGIN. Microcal (TM). Vers. 6.0. Computer software. Copyright Microcal Software Inc, 1990-1999.
22. WINDOWS, Statgraphics Plus. Vers. 5.1. Computer software. Statiscal Graphics Corp, 1997.
Recibido: 16/11/2017
Aceptado: 8/01/2018
Dra. C. María de los Ángeles Arada-Pérez, Departamento de Química. Facultad de Ciencias Naturales y Exactas, Universidad de Oriente, Santiago de Cuba, Cuba, mayarada@uo.edu.cu