Introduction
Enzymes are macromolecules synthesized inside all living cells, whose function is catalyze a large amount of biochemical reactions of paramount importance for sustaining life.1 They are biopolymers of proteinaceous nature with high rates of specificity and efficiency, refined by millions of years of evolutive selection.2 A feature that has attracted attention in recent years is the so-called "enzyme promiscuity", that is the potential ability of an enzyme to catalyze reactions that differ from those for which they have evolved.3 Among the most studied enzymes with this property, lipases outstand because of their demonstrated versatility with various organic substrates.4,5
Lipases (triacylglycerol hydrolases, EC 3.1.1.3) are a class of serine hydrolases belonging to α/β hydrolases superfamily6, which physiological function is to speed up the metabolism of lipids by catalyzing triglyceride transformation into long-chain fatty acids and glycerol. In vitro, they are employed as catalysts for different synthetic processes. The quota corresponding to lipases among the total volume of enzymes used in bio-catalysis with emphasis on organic synthesis reach 30 %.7
Even when lipases are widespread distributed in nature and can be found in animals, plants, bacteria, and fungal species, the latter are the preferred sources for the production of these enzymes. Approximately 50 % are obtained from filamentous fungi, because of the lipases secreted by these organisms are mostly extracellular, which facilitates the subsequent recovery and purification, reducing costs, in addition to being able to operate in a wide range of environmental conditions.8
In a previous work, 40 strains of filamentous fungi were isolated from residues of the vegetable oil industry and screened for the production of extracellular lipases.9 Four of these strains were selected to carry out the present study, in order to assess the extracellular lipolytic activity in submerged fermentation, in presence of soybean oil as only carbon and energy source.
Materials and methods
Fungal strains
Fungal strains used in this research were isolated from vegetable oil-contaminated materials, which were collected in the Oil Producer and Refining Company ECASOL, located at km 4½ of Mar Verde road, Santiago de Cuba.9 These strains showed high levels of extracellular lipase activity in liquid medium containing soybean oil as only carbon and energy source. The strains were labeled as follows: MS1-5, obtained from the sediment deposited on the walls of one of the drainage ditches of the of crude oil reception point; MS2-8, from oil wastes-contaminated soil coming from the pipelines of the crude reception point; ML5-32 and ML6-35 strains were isolated from wastewater samples, with high and low fat content, respectively, which runs through one of the drainage ditches located nearby the point of reception of the crude.9 Fungal strains were stored in YPDA slants at 4 ºC.
Culture media
YPDA medium was composed (in g/L) as follows: yeast extract 10, peptone 20, glucose 20 and bacteriological agar 15.
The basal lipase production medium was composed (in g/L) of: K2HPO4 2.5, (NH4)2SO4 1.3, urea 1,3, MgSO4·7H2O 0,5 and yeast extract 0,5, supplied with a soybean oil emulsion 20 % (v/v). The initial pH was adjusted to 6.0 with 1 mol/L NaOH. Urea was added to the sterile medium from 50X stock solution, which were sterilized by filtration through bacteriological filter (0,22 μm) and kept in amber bottle at -20 °C. The soybean oil emulsion was composed of 5 % (v/v) soybean oil emulsified in an aqueous 0,1 % (v/v) Tween 80 (Sigma) solution. Oil emulsion and basal medium were separately heat sterilized (121 °C for 20 min). Urea and oil emulsion were added to the mineral medium just before inoculation.
Lipase enzyme production in liquid medium
Fungi strains were cultivated in YPDA slants at 30 ºC until abundant sporulation was obtained (5-7 days). Spores were harvested with 5 mL of a saline detergent sterile solution (0,1 % Tween 80, 0,9 % NaCl) and this suspension was filtered through sterile glass wool to remove mycelium. Conical flasks of 200 mL, containing 20 mL of lipase production medium, were inoculated with fresh spore suspensions from each strain to obtain an initial concentration of 1·105 spores/mL. The inoculated flasks were incubated for 9 days in an orbital shaker, at a stirring speed of 150 rpm, at 30 ºC. Periodical culture samples were withdrawn at 1, 2, 3, 5, 7 and 9 days for the determination of biomass (as dry weight), pH, lipase activity and residual fat. Samples of the fungal cultures were centrifuged for 20 min at 10 000 rpm; the solid was employed to quantify the biomass, and the supernatant was taken to determine the pH and for estimating the residual fat and lipase activity.
Three independent experiments were performed.
Determination of lipase enzymatic activity
The determination was performed using an emulsion of vegetable oil in Arabic gum (AG) as substrate according to Colla et al.10 For the quantification of released fatty acids, the method of Marseno et al. was used, which is based on the colorimetric determination of the cupric complex formed by the fatty acids.11 In brief, 500 μL of 25 % (v/v) soybean oil emulsion in 7 % AG (m/v) was poured into glass test tubes fitted with a screw cap. To each tube, 50 μL of 0,4 mol/L acetate buffer (pH 4,8) or 0,4 mol/L phosphate buffer (pH 7,0) were added, according to the required pH for the enzymatic reaction, mixed well for 10 s in vortex and incubated at 37 °C for 10 min. Then, 250 μL of the enzyme crude (culture supernatant) were added, vortexed immediately for 10 s and incubated at 37 °C for 25 min. Then the enzymatic reaction was stopped by addition of 35 μL of 2 mol/L hydrochloric acid, which was mixed immediately in vortex for 10 s and cooling in an ice bath for 5 min.
Afterwards, 1 mL of isooctane was added, vigorously vortexed for 2 min and allowed to stand until the phases were separated (centrifugation was used when necessary). Then, 800 μL of the upper (organic) phase were carefully extracted and dropped into another glass tube containing 1 200 μL of isooctane. To this second tube, 400 μL of copper acetate-pyridine buffer [5 g of copper acetate are dissolved in 80 mL of water, the pH is adjusted to 6 using pyridine and the volume is completed to 100 mL] were added and vigorously stirred for 5 s in vortex. The mixture was allowed to stand for 10 min and the absorbance of the isooctane fraction was read at 715 nm using isooctane as blank. A standard curve of 1-5 μmol/mL of palmitic acid in isooctane was used. One unit of lipolytic enzymatic activity was defined as the amount of enzyme that releases 1 μmol of fatty acids per minute under the established test conditions.
Enzymatic assays were carried out in triplicate and the average values were calculated.
Analytical determinations
The biomass was determined by means of the gravimetric method, for which biomass was dried until constant weight at 80 ºC. The pH was measured at 25 °C in a digital pH meter provided with a combined electrode (Mettler ToledoTM Ingold Series).
The residual fat content in the culture media was estimated from the total fatty acids released after exhaustive alkaline hydrolysis in presence of alcoholic KOH.12 The fatty acids were quantified according to the colorimetric method described by Marseno et al.11 For the assay, aliquots of 0,5 mL of sample from well homogenized cell-free culture medium were added in test tubes fitted with a screw cap. Then 1,5 mL of 3 % (w/v) ethanolic KOH were added and homogenized in a vortex stirrer for 5 s. The tubes were heated for 1 h in a water bath at 80 °C. Afterward, the tubes were uncovered; 1 mL of distilled water was added, homogenized and heated at 80 °C during 10 min. Subsequently, 300 μL of 3 mol/L HCl were added, vortexed for 5 s and allowed to cool at room temperature. Then 2 mL of isooctane were added and vigorously vortexed for 2 min. Next, aliquots of the upper phase (isooctane) were taken and poured into tubes with isooctane so that appropriated dilutions were obtained for a final volume of 2 mL. Finally, 400 μL of copper-pyridine acetate buffer were added to the tubes containing the aforementioned dilutions, mixed at a vortex for 5 s and allowed to stand for 10 min, and the absorbance of the organic phase was read at 715 nm against an isooctane blank. A standard curve of 1-5 μmol/mL of palmitic acid in isooctane was used. The weight concentration of the residual fat was calculated assuming a saponification equivalent for the soybean oil of 290,9 g/mol, which was calculated on base of the average fatty acid composition reported for this oil.13
Analytical determinations were carried out in triplicate and the average values were calculated.
All the culture media components and reagents used in this research were from Uni-Chem (China) and Panreac (Spain), respectively, unless otherwise indicated.
Results and discussion
Kinetics of fungal growth and substrate consumption in submerged culture
Figure 1 shows the pH variations in the culture medium for the different strains, when they grew in mineral medium supplemented with soybean oil as only carbon and energy source. A significant decrease in pH from 6 to 4 units was observed, with the exception of the ML5-32 strain, where a slight rise occurred. The pH decreasing took place in parallel with the fat depletion, which was consumed by the fungal strains. The consumption of triglycerides is preceded by lipase-catalyzed hydrolysis, with the consequent release of the fatty acids and the acidification of the culture medium. Once the fatty acids were consumed, a gradual increase in pH is expected, as observed in almost all strains studied, due to hydrolysis of urea (spontaneous or catalyzed by urease enzymes). The unexpected behavior showed by ML5-32 strain could be related to a very much fast uptake and metabolic processing of fatty acids, with the consequent pH increase in the culture medium due to urea hydrolysis.

Fig. 1 Kinetics of fungal growth, substrate consumption and culture medium pH in mineral medium with soybean oil as only carbon and energy source
In all strains a continuous increase in biomass concentration concomitant with a progressive reduction in growth rate was observed, mainly from the fifth day of fermentation, which corresponds to the depletion of the substrate in the culture medium (figure 1). Biomass concentrations between 12 and 20 mg/mL were achieved, with growth yields between 0,65 and 1,08, which is satisfactory for the fermentative process, since the higher the biomass yield, the higher increase in enzyme production and productivity must be expected.
These results indicated that the composition of the culture medium and the source of carbon used (soybean oil) were adequate for satisfying the growth requirements of the studied filamentous fungus strains. These facts are in agreement with the origin of fungal strains, which come from soil and wastewater samples contaminated with soybean oil refinery residues. The strains with the best growth were MS2-8 and ML5-32, with biomass concentrations of 20 mg/mL in both cases. The microbial growth obtained for these strains was higher than those reported for the production of lipases by Aspergillus spp. (9,7-16 mg/mL) by Coca et al.14
Kinetics of lipase production
The extracellular lipolytic activity was assessed at pH 4,8 and 7,0 (figure 2). In a comprehensive review on the subject, Singh et al. stated that lipases of fungal origin work in a wide pH range.15 It have been referred that lipases produced by different strains of the genus Aspergillus exhibit optimal performance with pH values between 4,5-7,5.16 Li et al. thoroughly overviewed several aspects of lipases from the genus Penicillium; they summarized that the optimal pH range for most lipases of Penicillium spp. was between 5,0 and 9,0.17 These findings supported the evaluation of the two selected pHs in our research.
The strains showed two peaks of enzymatic activity (figure 2), which evidences the probable presence of at least two lipase isoforms. Table 1 shows the average enzymatic activity corresponding to these peaks. Many authors have demonstrated the existence of more than one lipase isoenzyme in several species of filamentous fungi and yeasts. Rhizopus species, such as Rhizopus niveus, are capable to express three isolipases with markedly different molecular weights;18Geotrichum candidum, produces two types of lipases with a notable contrast in their enzymatic activities;19 whereas Yarrowia lipolytica can potentially produce 25 lipase isoforms, according to a genomic analysis performed on this yeast.20 It is necessary to emphasize that in strain ML5-32, unlike the rest, the lipolytic activity corresponding to the second peak was higher to that of the first lipase (for both of pH values), which could be also indicative of the existence of more than one extracellular lipase. The selected strains with the highest enzymatic activity at 48 h were ML5-32 and ML6-35 (580 and 584 U/L, respectively) at pH 4,8, and MS2-8 (545 U/L) at pH 7,0 (table 1).
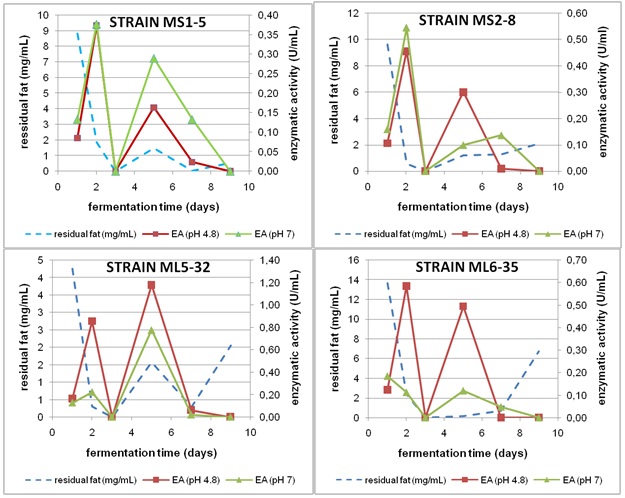
Fig. 2 Kinetics of substrate consumption and lipase production in mineral medium with soybean oil as carbon source. EA: Enzyme activity
Table 1 Enzymatic activity (U/L) in the peaks of maximum extracellular lipase accumulationa
| Strain | 48 h | 120 h | ||
|---|---|---|---|---|
| pH 4.8 | pH7.0 | pH 4.8 | pH7.0 | |
| MS1-5 | 374 ± 11 | 377 ± 26 | 164 ± 10 | 290 ± 9 |
| MS2-8 | 456 ± 14 | 545 ± 22 | 300 ± 21 | 99 ± 8 |
| ML5-32 | 580 ± 29 | 151 ± 9 | 730 ± 22 | 481 ± 34 |
| ML6-35 | 584 ± 23 | 112 ± 7 | 49 ± 1 | 119 ± 4 |
a Enzymatic activity values correspond to the average ± standard deviation of three independents experiments
This study contributes to a better understanding about the excretion of lipases by filamentous fungi in submerged culture, taking into account the quantification of lipolytic activity at two different pH values (neutral and acidic). To our knowledge no study targeting this issue has been reported yet.
It has been reported that the medium composition influence notably on lipase production in filamentous fungi when these are cultivated in liquid media. This is the reason for recommending a culture medium with a similar composition to the designed in this research when using oil vegetable as a carbon source. It is well known that its sole presence has the effect of inducing lipases production.21 Besides, several studies of optimization of culture medium through factorial designs experiments have shown that the presence of vegetable oils (olive, soybean, sunflower, etc.) had significant effects on lipases production.22,23
The appropriated levels of extracellular lipase enzymes obtained in this work under conditions of submerged culture allow us to continue with their study towards the search of optimal conditions of production, as well as the exploration of their potential applications.
Relationship between substrate consumption and lipase production
All the strains showed similar results respect to substrate consumption kinetics, which was completely exhausted at 72 h of culture (figures 1 and 2). Similarly, the peaks of lipolytic activity took place at 48 h and at 120 h, passing through a minimum at 72 h, when no enzymatic activity was detected. This intimate relationship between substrate consumption and the accumulation of lipases suggested that the first peak of enzymatic production corresponds to an enzyme isoform directly linked to the catabolism of the substrate. Therefore, it was associated with growth as occurs regularly for these enzymes, inducible by the substrate and most likely of extracellular origin. It have stated that there are metabolic processes in the cell focused on cell proliferation and growth, which includes all biochemical and intermediary routes (primary metabolites, like lipids) related to energy production, cell reproduction and viability. These intermediaries are produced in the active stage of growth or exponential phase in the microbial growth curve.24
After 72 h, the culture medium became transparent for all cases, which clearly evidenced the complete soybean oil consumption by microorganisms. However, the colorimetric assay, which was used to quantify fatty acids, indicated the presence of these compounds in the culture medium. From these observations it would inferred that the fatty acids detected at 120 h belong to compounds of the secondary metabolism of fungi and, therefore, it could be also assumed that the second peak of enzymatic activity (figure 2) corresponds to a second lipase isoform, which synthesis is associated to the secondary metabolism.
It have been stated that there are metabolic processes including secondary metabolism, in which produced compounds are commonly excreted to the cell environment without a vital importance. The biosynthesis of secondary metabolites generally occurs due to conditions of nutrients depletion in the environment, differentiation and sporulation25, which is in agreement with our results.
In the above-mentioned cases of synthesis of more than one lipase, the enzymatic activity values belonging to the first peak, at 48 h of culture, are more interesting in view of the potential industrial applications of the enzyme.
Conclusions
Four strains of lipolytic filamentous fungi were cultivated in submerged fermentation using soybean oil as only carbon and energy source. Biomass concentrations between 12 and 20 mg/mL were obtained, with growth yields between 0,65 and 1,08, indicating that the composition of the medium and the carbon source used were efficient for growth. The strains with the best growth were MS2-8 and ML5-32, with biomass concentrations of 20 mg/mL. During time-course of fermentation the lipolytic activity was evaluated at pH 4,8 and 7, and two peaks of enzymatic activity at 48 and 120 h were found. Selected strains with greater lipolytic activity at 48 h were the ML5-32 and ML6-35 (580 and 584 U/L, respectively) at pH 4,8 and MS2-8 (545 U/L) at pH 7,0. These results are the basis for future studies aimed at the optimization of the fermentative process for obtaining lipases, as well as the molecular and kinetic characterization of isolated and purified enzymes.














