Introduction
The use of medicinal plants is as old as the history of human beings. Plants with medicinal properties were first used empirically for the cure of diseases differentiating the ones that cured of those which killed. This knowledge passed down from generation to generation appearing in the ancient documents of every civilization, recording the heritage of those societies, which has traversed humanity until today.
Nowadays the aromatic plants are used to obtain secondary metabolites with high potential as antimicrobial agents. One of the plant families more explored with this proposed is Rutaceae. This is a cosmopolitan family, but mainly tropical and sub-tropical represented by 145 to 160 genres and from 925 to 1 800 species. This diversity it is also extended to the chemical compounds isolated in those species. A vast number of secondary metabolites such as alkaloids 1,2 limonoids, coumarins 3 and flavonoids are some of the most representatives.4 Additionally, several genres as Pilocarpus, Zanthoxilum and other are also producers of essential oils.5 With such diversity of compounds, it becomes in one of the most appreciable families from the medicinal potential point of view in the plant kingdom.6
One of the most popular genres of this family is Amyrissp. with several species well studied from it chemical and pharmacologic point of view due to its potential as source of new drugs. Species as Amyris balsamífera L and Amyris simplicifolia H. are high producers of saponins, terpenoids, tannins and coumarins.7 In Amyris plumieri DC were reported six chromomethyl amines with elevate potential as inhibitors of the enzymes of cytochrome P450 and as anticancer agents.8 Similar pharmacological profile was detected for the flavonoids isolated from Amyris madrensis W. With high cytotoxicity against prostate cancer cell cultures via microtubules depolymerization.9 Amides were other kind of metabolites isolated in Amyristexana W. demonstrating antimicrobial activity against Colletotrichum spp and Planktothrix perornata.10
Amyris elemifera L. is one the species of this genre that grows in Cuba. For this species there is not found any scientific information consisting it chemical nor pharmacological profile. That is why the present study pretends to offer a first approach of the chemical composition and antimicrobial activity of extracts prepared from the leaves of this plant.
Materials and methods
Plant collection and processing
Leaves from Amyris elemifera L. plant were collected in February 2018 at "el Palenque" in the Siboney community located in the municipality of Santiago de Cuba. A plant sample was taxonomically identified by specialists of Vegetal Biology at "Universidad de Oriente" and settled at the herbarium of this institution with the registration number 2350.
Collected leaves were divided in two halves. The first half (in fresh condition) was designated to the extraction of their volatile compounds by hydrodistillation-cohobation extraction method, using a Clevenger equipment for 3 h. Essential oil was dried using anhydrous sodium sulfate and stored under refrigeration at 8 ± 2 ºC, in amber glass bottles until the bioassays could be conducted. The oil yields were calculated and expressed as a percentage related the volume of the essential oil collected versus the total weight of fresh leaves extracted (v: w). The second half was dried on the shadow (considering their aromatic character) at room temperature until constant weight. Once dried, it was milled in a blade mill (MRC Model KM 700, Germany) to diminish the particle size facilitating the further extractive process.
The residual wet of the leaves powder was determined by the infrared gravimetric methodology. In brief: In Petri dish 5 grams of the leaves powder were weighted and placed on a MB-110 balance connected to an infrared lamp regulated at 105 ºC. The loss of weight was monitored until becomes constant, calculating the residual wet expressed in percent. This process was realized three times.
Extract preparation and fractioning
Two hundred and fifty grams of dried leaves were placed in a percolator and ethanol 95 % was added as solvent extraction for 24 h. After this time, solvent was removed and replaced with new quantity of ethanol 95 %, macerating for other 24h. This procedure was repeated by 7 days until the solvent looks colorless. All the extracts were joined and vacuum concentrated in a KIRKA-WERKE Rotary Evaporator (Germany) reducing the final volume to 250 ml, obtaining final concentrations equivalent to 1 g/mL (dry leaves weight).
This raw extract was fractioned using a successive liquid-liquid partition with three pure solvents with high difference of polarities: n-hexane, ethyl acetate and n-butanol. By this way three different fractions were obtained, being concentrate until syrup by the same conditions that the raw extract for the further experiments.
Qualitative chemical composition
Chemical reactions established in the phytochemical screening technique described in the literature were performed to define the metabolites or groups of metabolites present in the raw extract as well as the three fractions obtained.11 For this purpose, dry fractions were solved in ethanol 95 % until reach a final concentration equivalent to 10mg/mL. The metabolites determined were: Phenols and Tannins, Alkaloids, Flavonoids, Triterpenes and Steroids, Coumarins, Quinones, Saponins, Reducing Sugars, and Amino Acids.
In vitro antimicrobial evaluation
The in vitro antimicrobial activity of the raw extract, the three fractions generated in the fractioning process (n-hexane, ethyl acetate and n-butanol) as well as for the essential oil were determined by the microdilution method with resazurin (redox indicator) in sterile 96-well microplates.13 The antimicrobial activity was tested facing the extracts to two bacteria, one yeast and three parasite strains supplied by the Laboratory for Microbiology, Parasitology and Hygiene (LMPH), University of Antwerp, Belgium. The microorganism used were Staphylococcus aureus ATCC 6538, Escherichia coli ATCC 8739, Trypanosoma cruzi (Tulahuen CL2, β galactosidase strain (nifurtimox-sensitive)), Leishmania infantum MHOM/MA (BE)/67, and Trypanosoma brucei Squib 427 (suramin-sensitive). Doxycyclin, Norfloxacine, Flucytosine, Benznidazol, Miltefosine and Suramine all from Sigma-Aldrich, USA were used as reference drugs for each one of the microorganism tested respectively. At the same time, the cell viability on MRC-5 model (human lung fibroblasts) was assessed as well as in PMM (Primary macrophages of mouse) cell culture to determine in this specific case the selectivity on L. infantum parasite. Tamoxifen was used as standard drug for the cell culture experiment. Both cell lines were purchased from ATCC (American Type Culture Collection). The concentration tested for all the extracts were those established by the host laboratory consistent in 128, 32, 8, 2 and 0.5 µgmL-1 for both, the antimicrobial and cell grow culture experiments.
Specific grow conditions were considered to guarantee the satisfactory grows of bacteria, yeast, parasite and cellular lines, according to the standards procedures established in the Laboratory for Microbiology, Parasitology and Hygiene (LMPH).12
For all cases the selectivity index (SI) allowed examining the relationship between cytotoxicity and a chosen activity. The SI was defined as the ratio between the CC50 value for cytotoxicity and the IC50 value for antimicrobial activity. Samples with an SI value of 10 or more were considered highly selective.
Results and discussion
Plant collection and processing
From the fresh leaves half was extracted a dense essential oil with an almost transparent color and with a yield equivalent to 0,54 percent who can be considered as acceptable according to the literature.13 On the other hand, the dried leaves reach the constant weight at the day number 17 having a residual wet equivalent to 11 % which can be considered as good and qualified as normal for non official plants according to the standards established in Cuba.14
Qualitative chemical composition
The results of this screening to the raw extract as well as the three derived fractions are shown in table 1.
As can be observed in this table 1, it highlights that eight of the nine determined metabolites turned out positive to the specific chemical reactions. For this extract phenols and tannins, alkaloids, flavonoids, triterpenes and steroids, coumarins, quinones, reducing sugars, and amino acids were positive. On the other hand, the n-hexane fraction results positive to fourth of the nine determined metabolites, but one of them proved to be dubitable (triterpenes and steroids). This fraction was positive to phenols and tannins, alkaloids, flavonoids, and coumarins. Triterpenes and steroids result positive at one (Lieberman-Burchard) of the two assay performed. Considering the non-polar characteristic of the hexane fraction, two results emerge as remarkable: the dubitable presence of Triterpenes and steroids and the strong evidence of the presence of phenols and tannins. In the first case and according to the lipophilic characteristic or triterpenes and steroids, those compounds should be extracted in a low polar solvent as hexane with the exception of those that appears as glycosides. Steroids and triterpenes in glycoside form have been informed for some species of the family rutaceae or genre Amyris15, therefore this can be the kinds of triterpenes and/or steroids present in A. elemifera leaves. On the other hand, phenols trend to be soluble in medium and high polar solvents due to the acidic characteristic of the phenol moiety. Nevertheless, some of them appear as substituent in some complex aromatic nucleus and or in polymer chains as occurs with the catequins.16 In those cases, the phenol compounds can be extracted with non- polar solvents as seem as happen in this study. Some non-glycosylate flavonoids can also be extracted in this kind of solvent and reach with the Ferric Chloride reagent.
Table 1 Kind of metabolites detected in the raw extract and it both fractions obtained from Amyris elemifera leaves
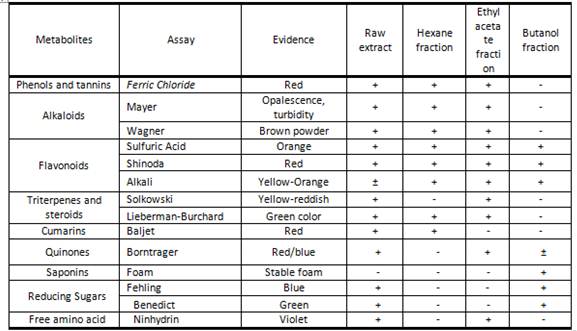
Legend: (+) Positive result. (-) Negative result (±) Dubitable result
Ethyl acetate fraction gives positive to six kinds of compounds. As medium polarity solvent, usually extract non polar compounds that appear in form of glycosides or with highly number of hydrophilic substituent. Is exactly what it happens with Steroids and triterpenes that is the kind of compounds that were extracted in this solvent instead the more non-polar hexane. Alkaloids and phenols are the other two metabolites that are in common with the hexane fraction with the only difference that in this extract the intensity (color followed) of the chemical reaction looks like more intense, meaning more concentration of these metabolites. Quinones appears in the plant kingdom as medium polar compounds, usually linked to one unit of monosaccharide, that is why it presence in this fraction was expected. Flavonoids also appear in this phase as well as in the other phases and extract. This solvent has been declared as the optimal for the extraction of this kind of compound, while the last kind of metabolite was the organic amino acid and amides that by it partial ionic charges (mainly amino acids) are extracted in medium and polar solvents.
The butanol fraction shows the presence of other three metabolites having in common with the raw extract, hexane and ethyl acetate fraction the flavonoids. This kind of metabolites widely spread around the plant kingdom, can exist under diverse pattern of substitutions since in form of polymer of cathequins and/or with highly degree of methoxylation instead their phenol groups (fitting with low polar solvents) until aglycone monomers highly oxidated and/or linked to one or more monosaccharide units (fitting with polar solvents). That is why is not rare the appearance in the three fractions. According to the color developed in the qualitative tests, looks like the hexane fraction extract isoflavones and proanthocyanins while ethyl acetate and butanol fractions extracts flavones, flavanones and chalcones. Other metabolites in the butanol fraction are saponins, reducing sugars and a dubitable result for quinones. All these metabolites are polar; therefore, it looks logic their presence in this fraction and not in the hexane one. Quinones present a strong positive result in ethyl acetate, therefore; it dubitable presence can be associate to a low concentration.
In general, this phytochemical study allows us to get an idea not only of the composition of A. elemifera extracts but also the chemical nature of the metabolites. That is the case of the alkaloids which are extracted only in the non-polar and medium polar fractions, therefore it is inferred that they appear in their base state and not in form of salt as also can occurs. This characteristic is consistent with the chemotaxonomic profile of rutaceae family in which bulky alkaloids, mainly derivates from the benzylisoquinoline pathway.17
In vitro antimicrobial evaluation
The number of drug resistant microorganism is nowadays a global phenomenon, that is why the studies searching for new antimicrobials are always welcome, especially if comes from medicinal plants.
The results obtained in this screening (table 2) reveals that the raw extract, the hexane and ethyl acetate phases and the essential oil are good anti-parasitic substances, presenting inhibitory concentration with values under 20µgmL-1. None of the extracts exhibited activity against bacteria and yeast. The butanol phase is inactive against all microorganisms. Making a qualitative correlation between the chemical composition determined and the antimicrobial activity emerges as possible metabolites responsible for the activity the alkaloids, triterpenes and steroids and phenols and tannins.
Table 2 Antimicrobial activity of raw extract and fractions of A. elemifera leaves
| Microorganisms | Raw extract IC50 (µgmL-1) | hexane IC50 (µgmL-1) | Ethyl acetate IC50 (µgmL-1) | Butanol IC50 (µgmL-1) | Essential Oil IC50 (µgmL-1) |
| Trypanosoma cruzi | 4,3 | 7,1 | 8,08 | >64,0 | 4,32 |
| Lehismania infantum | 19,0 | 8,1 | 6,78 | >64,0 | 19,03 |
| Trypanosoma brucei | 4,0 | 2,3 | 3,17 | >64,0 | 4,03 |
| Staphyloccocus aureus | >128,0 | >64,0 | >64,00 | >64,0 | >128,0 |
| Escherichia coli | >128,0 | >64,0 | >64,00 | >64,0 | >128,0 |
| Candida albicans | >128,0 | >64,0 | >64,00 | >64,0 | >128,0 |
The activity of alkaloids against diverse parasite strain has been reported, especially for Trypanosoma sp.18 Previous studies on the family species reveals that son species of Zanthoxylum genre trends to be active against several microorganisms, particularly the non-polar phases.19 Similar behavior is also found in Pilocarpus and Citrus genres.20 Something similar occurs with glycosylated steroids as stigmasterol-3-O-β-D-glucopyranoside and sitosterol-3-O-β-D-glucopyranoside who have demonstrated good antiparasitic activity 21 and some polyphenols as.22
With a different chemical profile by its own condition, the essential oil also exhibit good anti-parasite activity. Once against neither antibacterial nor yeast activity was found. Even when no chemical analysis for this extract was done, the methodology used to extract the essential oil usually extracts volatile compounds derivates from mono and sesquiterpenes, and in some cases some diterpenes and some phenylpropanoid derivates. All those compounds have proved to be good anti-parasites.23
Selectivity indexes
In parallel to the antimicrobial activity the cell grow inhibition test was performed in order to established the selectivity of the extract tested over the microorganism in favor to the eukaryotic cell characteristic of the infection host. The PMM cell line is specific for Leishmania infantum in amastigote stage while MRC-5 cell line was used for the rest of the microorganisms. In table 3 are present the half cytotoxic concentration CC50for each extract, while in table 4 is presented the selective index calculated for each microorganism.
Table 3 Cytotoxic concentration (CC50) calculated for each extract of A. elemifera leaves
| Cell Line | Raw extract | hexane | Ethyl acetate | Butanol | Essential Oil |
| MRC-5 | 11,78 | 6,15 | 8,08 | >64,0 | 16,00 |
| PMM | 16,00 | 8,00 | 32,00 | >64,0 | 64,00 |
Table 4-Selectivity indexes calculated for each microorganism and extract of A. elemifera leaves
| Microorganisms | Raw extract | hexane | Ethyl acetate | Butanol | Essential Oil |
| Trypanosoma cruzi | 2,73 | 0,86 | 1,00 | - | 3,70 |
| Lehismania infantum | 0,84 | 0,99 | 4,72 | - | 3,36 |
| Trypanosoma brucei | 2,94 | 2,67 | 2,55 | - | 3,97 |
| Staphyloccocus aureus | - | - | - | - | - |
| Escherichia coli | - | - | - | - | - |
| Candida albicans | - | - | - | - | - |
A simple inspection to table 4 denotes that with the exception of the selectivity index calculated for Lehismania infantum in the ethyl acetate phase that classified as partially selective, the rest of selective indexes are no good, meaning that the anti-parasitic activity courses with an unspecific way killing both microbes but also the host cells. Quinones and amino acid are the two kinds of compounds that are extracted only in ethyl acetate phase, and they can excerpt some activity but not toxicity to the cell host. Nevertheless, this hypothesis should be proved in other antimicrobial screening test.
Conclusions
Of the essential oil a yield of 0,542 % was obtained, value can be considered as an average yield when compared with other species of the same genus. The ethanol extract as well as it derivate fractions shows the present of Phenols, Alkaloids, Flavonoids, Triterpenes and steroids, Cumarins, Quinones and amines. From them, Phenols, Alkaloids, and Triterpenes and steroids looks like the responsible for the good anti-parasitic activity demonstrated. Nevertheless, it was obtained an acceptable selectivity index only for the ethyl acetate phase when faced to Lehismania infantum opening a gate to deeper future studies in the long process that means to get new drug candidates














