Introduction
The study of the prospects for the use of highly porous polymeric materials in various areas of human activity is being actively conducted by many scientists. A highly porous polymeric material can be obtained by polymerizing highly concentrated W/O emulsions, containing a monomer or mixture of monomers as the organic phase. After polymerization and removal of the aqueous phase, a porous structure is formed, which is similar in structure to the initial emulsion.1,2
The size of water droplets in a highly concentrated emulsion, and hence the pores of a highly porous polymeric material, can be controlled by changing the homogenization rate during the emulsion preparation, the composition of the emulsion (for example, by varying the surfactant concentration used to its stabilization3, the intensity of Ostwald ripening in emulsions when electrolytes are added in the dispersed phase.4
Many authors have confirmed the possibility of using highly porous polymeric materials for cell engineering as a framework for tissue repair and cell growth 5,6, containers for creating carbon structures and nanoparticles 7, catalysis 8,9, separators in Li-ion batteries 10,11, carbon dioxide sorption 12, and water purification from various pollutants.13,14
The desired properties of highly porous polymers can be enhanced or even new properties can be given to them by creating a composite material, when introducing various types of nanoparticles into the polymer framework. For example, the introduction of TiO2 nanoparticles improves the mechanical properties and affects the morphology of the highly porous polymer 15, but also increases its sorption characteristics with respect to carbon dioxide.16
The possibility of using silicon nanoparticles to create a highly porous composite polymer material was discussed in.17) Authors studied in detail the mechanism for the extraction of nickel ions from solutions and positive results were obtained with the introduction of silicon nanoparticles into the polymer matrix.
Among the fillers for highly porous polymeric composites, iron oxide nanoparticles are preferred due to their availability, ease of preparation, and magnetic properties.
Iron oxide nanoparticles easily undergo surface modification with various functional groups, which may also have a significant effect on the properties of the composite. Thus, the effect of modifying the surface of a mixture of magnetite nanoparticles and maghemite on the morphology and thermal properties of a highly porous composite was shown 18, and the composite material demonstrated a high sorption capacity with respect to Hg(II) when Fe3O4 nanoparticles, coated with humic acid, were incorporated.19
Some authors 20,21 improved sorption properties of the polymeric composite with magnetite nanoparticles, when removing antibiotics, Pb2+ and Cd2+ ions from aqueous solutions. Including magnetite nanoparticles had an additional positive effect - the stability of highly concentrated emulsions before polymerization increased.
The magnetic properties in a highly porous polymeric composite are especially important when it is used as a sorbent for cleaning water bodies from oil pollution. Magnetic properties allow facilitating its recollection from the water surface after the end of the sorption process.22
Among iron oxides, γ-Fe2O3 is of interest for this application. γ-Fe2O3 nanoparticles are resistant to oxidation, their surface can be hydrophobized, which is useful for sorption of oil products.23 Also, it is possible to recollect the nanoparticles using an external magnetic field.24
The aim of this work is to prepare highly porous polymeric composite materials, based on a poly(styrene-co-divinylbenzene) [poly (St-DVB)]. γ-Fe2O3 nanoparticles, modified with oleic acid, are incorporated into the highly porous polymer. These materials are studied during sorption of oil products from water surface.
Materials and methods
Reagents and materials
The following reagents were used: monomers - styrene (≥ 99 %) and divinylbenzene (≈ 55 %), surfactant - sorbitan monooleate - Span 80 (≥ 95 %), polymerization initiator - ammonium persulfate (≥ 98 %). All reagents, produced by Sigma-Aldrich, were not subjected to additional purification. Polymerization inhibitors were not removed from styrene. The following reagents were used to obtain maghemite nanoparticles: iron(II) chloride tetrahydrate (Acros Organics, ≥ 99 %), iron (III) chloride hexahydrate (Acros Organics, ≥ 99%), ammonium hydroxide (CHIMMED, ≥ 99 %), nitric acid (CHIMMED, ≥ 99 %), oleic acid (CHIMMED, ≥ 98 %); reagents were not subjected to additional processing before synthesis. Bidistilled water was used in all experiments.
Synthesis of γ-Fe2O3 nanoparticles
γ-Fe2O3 nanoparticles were obtained by the coprecipitation method. A 13,3 M aqueous solution of ammonia was added to an aqueous solution of a mixture of FeCl2 and FeCl3 until a pH of ~10-11 was reached. The pH was monitored with a pH-meter (SevenEasy, Mettler Toledo). Oleic acid was added to the resulting solution and mixed at 400 rpm using an overhead stirrer (IKA EUROSTAR power control-visc P1) for one minute. Then, a 6.7 M aqueous solution of nitric acid was added until pH 5. The nanoparticles, with oleic acid adsorbed on their surface, formed loose aggregates and settled on the bottom of the vessel. The precipitate was washed with distilled water 3-4 times and dried at room temperature in a desiccator.
Preparation of highly concentrated W/O emulsions
Styrene, divinylbenzene and sorbitan monooleate were placed in a vessel for the preparation of the emulsion. Magnetic nanoparticles powder was added to the mixture of monomers and surfactant. A 6 mM ammonium persulfate solution was added to the mixture at a rate of 3 ml/min with constant stirring using an overhead stirrer (IKA EUROSTAR power control-visc P1) at a speed of 1200 rpm 3
Production of highly porous polymeric composites with magnetic nanoparticles
Highly concentrated emulsions were placed in an L9/11/SKM Nabertherm oven and kept at 65 °C for six hours to polymerize and dry the samples.
Investigation of the structure of magnetic nanoparticles and highly porous polymeric materials
The average size of γ-Fe2O3 nanoparticles was determined by transmission electron microscopy (TEM) using a microscope (JEOLJEM-1011, Jeol Ltd.).
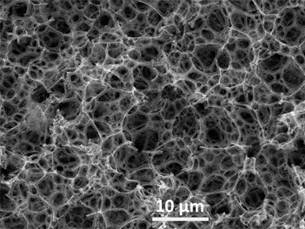
The structure of a highly porous polymeric composite was studied using a scanning electron microscope (SEM) (JSM 6510 LV, Jeol Ltd.).
The micrographs obtained by TEM and SEM were used to determine the size of γ-Fe2O3 nanoparticles and the pore diameter in the highly porous polymeric nanocomposite.
Results and discussion
Structure and pore size of highly porous polymeric nanocomposite
For effective sorption of oil products, porous polymeric materials should have high porosity. With an increase in the proportion of pores in the volume of the material, the interlayer between the pores becomes thinner 3, which can affect the mechanical characteristics of the material, including the strength. As shown by our previous studies, porosity of 95 vol % is optimal.25 With such porosity, the sorbents exhibit a high sorption capacity and retain their structure intact during the entire process of sorption of oil products from the water surface.
For obtaining a polymeric nanocomposite, the size of nanoparticles incorporated into polymer layers should not exceed their thickness. In this case, a uniform distribution of nanoparticles inside the polymer interlayers and on the inner surface of the pores is achieved. At the same time, the destruction of the porous structure in the process of polymerization of the dispersion medium of emulsions does not take place.
Figure 1 shows an image of synthesized γ-Fe2O3 nanoparticles coated with oleic acid, obtained by TEM, and nanoparticle size distribution. The average size of nanoparticles was 12 ± 2 nm. The composition of γ-Fe2O3 nanoparticles was confirmed by XRD analysis. 26
To characterize the structure of the obtained poly (St-DVB) composite nanomaterials, images were obtained using SEM (figure 2). The size of the polymeric layers in this material was 70-150 nm. The size of γ-Fe2O3 nanoparticles was significantly less than the interlayers thickness; therefore, they were incorporated into interlayers, without leading to their destruction.
It should be noted that in materials of this kind, an interconnected system of open pores of two types is obtained.27 The first type of pores consists of pores-voids, obtained by removing the dispersed phase of the original W/O emulsion during the drying process. The second type is the pores-throats of smaller diameter in the polymer interlayers, connecting large pores-voids. The result is a network of interconnected open channels occupying the entire volume of the porous polymer. This allows oil products to fill the entire pore space, reaching maximum saturation.
Secondary openings can be formed due to the occurrence of polymer-rich areas and surfactant-enriched areas in polymer interlayers in the course of the polymerization process. 28 The zones enriched with surfactants may appear as small droplets in the polymer interlayer, and then increase in size as the polymerization proceeds. Such areas break through when drying porous polymeric material.3
The average sizes of pores and throats in samples of highly porous poly(St-DVB) composite materials with magnetic nanoparticles are presented in table 1. The concentration of magnetic nanoparticles was 0 wt.%, 15 wt.% and 25 wt.% from the mass of monomers. It can be seen that the inclusion of γ-Fe2O3 nanoparticles has practically no effect on the internal structure and pore size in highly porous poly(St-DVB) composite.
Sorption properties of highly porous poly(St-DVB) composite
When cleaning the surface of water bodies from oil products in case of emergency spills, the sorbent simultaneously contacts with oil products and water. For effective sorption of oil products, the surface of the sorbent must be highly hydrophobic in order to adsorb water slowly and in small quantities. At the same time, the sorption rate of oil products should be high, in order to avoid the formation of stable oil emulsions and their subsequent deposition on the underwater soil.
A photograph of a water drop placed on the surface of high porous poly(St-DVB) polymer and a composite with γ-Fe2O3 nanoparticles is shown in figure 3.

Fig. 3 A drop of bidistilled water on the surface of poly(St-DVB). γ-Fe2O3 nanoparticle concentration: 0 wt.% (a), 25 wt.% (b)
The wetting angle with water was 144° for a polymer that did not contain magnetic nanoparticles, but it increased up to 154° after adding 25 wt.% γ-Fe2O3 nanoparticles. Therefore, hydrophobicity of the poly(St-DVB) composite improved when γ-Fe2O3 nanoparticles were incorporated into the porous polymer.
The properties of poly(St-DVB) composite samples were studied by sorption of kerosene, transmission oil and bidistilled water. In order to determine the sorption characteristics of sorbents with respect to oil products with different viscosities, the sorption of mixtures of kerosene and transmission oil in various volume ratios was also studied (table 2).
Table 2 Properties of liquids sorbed by poly(st-dvb) composite
| Liquid | Viscosity of liquid, mPa.s |
|---|---|
| Bidistilled water | 1,0 |
| Kerosene | 1,5 |
| Kerosene and transmission oil in volume ratio: 1:1 1:3 1:9 | 3,0 6,0 14,7 |
| Transmission oil | 671,0 |
For the determination of the sorption characteristics, the highly porous polymeric material was placed on the surface of water or oil. In all cases, the volume of water or oil product was several times larger than the volume of porous polymer, taking into account the pore space.
Figure 4 shows, as an example, the sorption curves of transmission oil and water with a polymer that does not contain magnetic nanoparticles and with 25 wt.% γ-Fe2O3 nanoparticles. The amount of adsorbed oil or water is expressed in grams per 1 g of sorbent. It can be seen from the above dependences that the addition of 25 wt.% nanoparticles sharply reduces the amount of water adsorbed in five minutes from 16,4 g/g to 1,4 g/g. At the same time, the amount of transmission oil adsorbed in five minutes increases from 17,5 g/g to 20,0 g/g.

Fig. 4 Kinetic curves of sorption of transmission oil and water of highly porous poly (St-DVB) without magnetic nanoparticles and with 25 wt.% γ-Fe2O3 nanoparticles
Magnetic nanoparticles were coated with an adsorption layer of oleic acid, which is a slightly polar substance. When such nanoparticles were incorporated into the structure of a highly porous polymer, its surface hydrophobicity increased; this led to a sharp decrease in water sorption.
Based on the kinetic sorption curves, similar to those shown in figure 4, the sorption rates of oil products and water were calculated in the initial period of time. Figure 5 shows data on the sorption rate at the initial period of time, depending on the viscosity of the substance to be adsorbed.
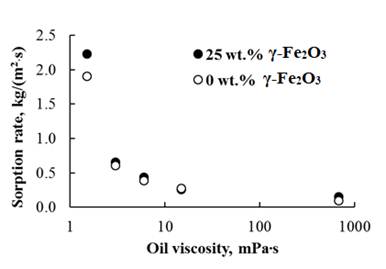
Fig. 5 Sorption rate of oil products with different viscosity of highly porous poly(St-DVB) polymer without magnetic nanoparticles and with 25 wt.% γ-Fe2O3
The sorption rate in the initial period of time decreased markedly with an increase in the viscosity of oil products from 1,5 mPa.s to 671 mPa.s. In the sample with 25 wt.% γ-Fe2O3 nanoparticles, the initial rate decreased from 2,23 kg/(m2·s) to 0,15 kg/(m2·s).
At the initial time, the sorption rate of oil products differed slightly for samples containing magnetic nanoparticles and without them. A significant difference was observed only in the sorption of kerosene. The initial sorption rate in the sample with 25 wt.% γ-Fe2O3 nanoparticles was higher than in the sample without magnetic nanoparticles. The hydrophobicity of the sample surface with γ-Fe2O3 nanoparticles was higher due to the presence of oleic acid; therefore, kerosene, having a low viscosity, penetrated the pore space more quickly.
Figure 6 shows the dependences of the sorption rate of transmission oil and water on the concentration of γ-Fe2O3 nanoparticles in poly(St-DVB) composite.
It is evident that with an increase in the concentration of γ-Fe2O3 nanoparticles, the sorption rate of the transmission oil almost linearly increased (Pearson’s r = 0,904). Along with that, the sorption rate of water decreased linearly (Pearson’s r = -0,976), which is associated with an increase in the hydrophobicity of the surface of the composites, since the magnetic nanoparticles were covered with an adsorbed layer of oleic acid. When the concentration of nanoparticles γ-Fe2O3 is 25 wt.%, the sorption rate of the transmission oil is more than 80 times higher than the water sorption rate.
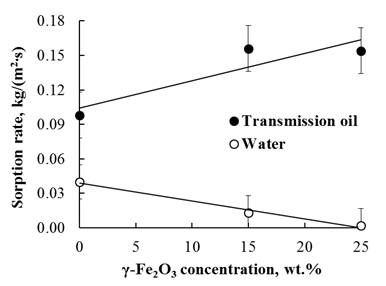
Fig. 6 Dependences of the sorption rate of oil and water on the concentration of nanoparticles of γ-Fe2O3 in the polymeric composite
As shown by our previous studies using poly (St-DVB) composite with Fe3O4 nanoparticles 29, the sorption rate of seawater is even lower than the sorption rate of bidistilled water. Therefore, a highly porous composite nanomaterial with magnetic nanoparticles is promising to eliminate oil spills from the surface of marine waters, including off the coast of Cuba.
Conclusions
A highly porous polymeric composite is obtained on the basis of poly(styrene-co-divinylbenzene) during the polymerization of dispersion medium in a highly internal phase emulsion. Highly porous nanocomposites with a γ-Fe2O3 nanoparticle content up to 25 wt.% based on the weight of monomers are investigated.
The study of the sorption properties of poly (St-DVB) composite shows that the addition of γ-Fe2O3 nanoparticles significantly reduces the initial sorption rate and the amount of adsorbed water. The sorption rate of oil products increases with increasing concentration of magnetic nanoparticles. The results indicate that poly (St-DVB) composite with γ-Fe2O3 nanoparticles is promising for use as a sorbent for cleaning the surface of water bodies from oil products during emergency spills