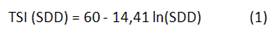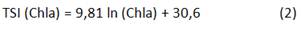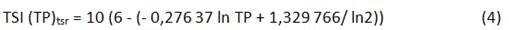Introduction
Eutrophication is a worldwide problem that affects not only the quality of water ecosystems but their services, because of the implications in their ecological balance and their environmental vulnerability together with natural climatic disturbances. The elimination of this problem becomes difficult or even impossible, being one of the main environmental challenges.1,2 The eutrophication is greatly accelerated by human activities like urbanization, industrialization, and agriculture; triggering the proliferation of planktonic cyanobacteria and toxic events.3,4
Artificial reservoirs have been extensively built-in Latin American and in the Caribbean region, modifying natural aquatic ecosystems to solve human needs. These are used for different purposes, being the anthropogenic eutrophication linked with excessive nutrient inputs from domestic wastewater, agricultural activities and urban runoff, the main threat that different water reservoirs worldwide face, particularly in tropical and subtropical water bodies, due to the population increase and a still poor sewage treatment infrastructure.5,6
In Cuba, specifically at the Eastern region, some studies were conducted in relation with the phytoplankton dynamic in artificial reservoirs since 2001, mainly in dry season, identifying the cyanobacteria presence in three water reservoirs, which benefits more than 80 % of the Santiago de Cuba population: Chalóns, Parada, and Charco Mono. In those studies, at least nine toxic cyanobacteria were identified: Microcystis viridis, Oscillatoria chalybea, Oscillatoria limosa, Oscillatoria tenuissima, Anabaena torulosa, Planktothrix sp., Lyngbya sp., Synechococcus sp. and Gomphosphaeria sp., being Microcystis spp. the most frequent species.7 The phytoplankton density range between 5 to 25 x104 cell.mL-1.7,8 The authors suggest that more researches need to be conducted in order to study the relationship of these events with eutrophication.
Traditionally the water quality in lakes and reservoirs has been assessed using limnological methods and laboratory analyses of field-sampled data, being time and cost consuming.9 One of the most important methods to evaluate and even classified waters is the determination of the trophic state by means of the Trophic State Index (TSI). It is an important property of the aquatic ecosystems which reflects the anthropogenic influence on water quality and ecological functioning, providing insight on how nutrient and light availability controls phytoplankton development.
Even when TSI is an important tool to evaluate the trophic state of aquatic ecosystems, some inconsistencies highlight when different papers are reviewed. The most used index since 1977 is Carlson’s (TSIa), explaining their close relationship with cyanobacteria growth. This index has been frequently used by researchers and government institutions to indirectly estimate the algal biomass and indicate the eutrophication degree of lentic ecosystems.10 However, the relationships and the equations for calculating the index should be adapted when applied to aquatic systems different from those from Carlson’s study, otherwise, they can lead to misconceptions when proceeding a trophic status assessment.6
Carlson index and the corresponding classification criterion was developed considering highest productive seasons in temperate lakes (spring and summer), but it is well known that tropical and subtropical ecosystems may have high primary production thought all the year 6; this index considers an empirical relationship between Secchi disc depth (SDD), chlorophyll a (Chla), and total phosphorus (TP).11 But the trophic state limits established for temperate systems are not suitable for tropical/subtropical reservoirs and may overestimate their enrichment condition 6 and other index and classification criteria highlight, considering the inconsistency to use the index developed to temperate systems in tropical/subtropical reservoirs.6
In this context, Salas and Martino (1991) proposed a simplified TP model and a trophic state classification for warm-water tropical lakes, based on the geometric means of TP and Chla concentrations (12 and Cunha et al. proposed an index (TSItsr) based on the annual geometric mean concentrations of TP and Chla 6 and developed considering experimental data obtained in tropical and subtropical reservoirs (not lakes) from Brazil.
It is well known that tropical and subtropical aquatic systems have specific sensitivities to eutrophication 13 because they are submitted to different climatological conditions and uses conflicts. Specifically, in the eastern region of Cuba, the climatic conditions are typical of tropical ecosystems, but insularity and Mediterranean conditions influence this area as well as the orography, where highlight mountain systems. Main seasons in Cuba are dry and rainy, but the eastern region has higher water and air temperatures (around 19,75 ± 0,48 and 34,25 ± 0,63 °C), respectively as well as high light irradiance.14,15 In this region water supply is the main use, highlighting the lack of trophic status data availability.
In this paper, the trophic status of twenty-four reservoirs located in the eastern region of Cuba was evaluated, analysing the relationship with cyanobacteria occurrence, identifying distinctive attributes in relation to the eutrophication levels.
Materials and methods
Description of studied area
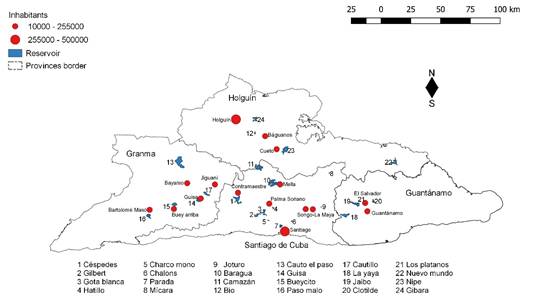
Twenty-four water reservoirs of the eastern region of Cuba, located in the provinces of Santiago de Cuba (10), Holguín (5), Granma (5), and Guantánamo (4) were sampled during September, 2015 to April, 2017 (figure 1). These reservoirs belongs to different basin and microbasin systems; having different capacities (ranged between 2, 38 to 330, 00 x 106 m3) being the main uses: crop irrigation and water supply for domestic and industrials purposes, benefiting around 3 625 800 inhabitants in the eastern region of Cuba.
Data collection and analysis
TP (mgL-1), Chla concentrations (µgL-1) and SDD (m) were measured. Data collection was limited to surface water samples (first 50 cm of water column). Four location were established in each reservoir. Three measurements were done in each location.
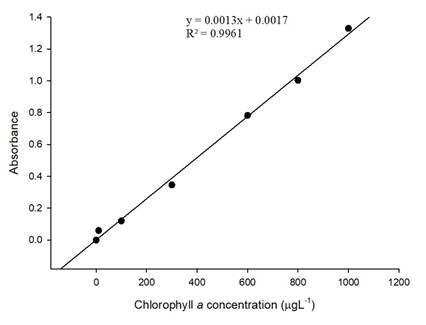
Chlorophyll a was measured in situ using an AquafluorTM handheld fluorometer (Turner Designs, USA) previously calibrated with chlorophyll a standard (Sigma) (Chlaf). Adjustment to express results of chlorophyll a in vivo as µgL-1 was made determining a correction factor, after the development of a calibration curve, which was prepared using serial dilutions from a standard Chla solution in methanol 90% (10 to 1000 µgL-1).16 It was developed measuring the absorbance (A) at 675 nm, using a spectrophotometer UV-vis (Genesys, USA). The concentrations of the samples were determined considering the resulting curve equation.
To determine TP concentrations, samples were collected in triplicate in acid-prewashed leak-proof polyethylene sample bottles into the first 50 cm of water column; sample bottles have a double seal to ensure safe transportation; they were maintained at 4 ºC until analyses with a maximum storage time of 2 days. TP was determined spectrophotometrically with a Genesys 10 UV-vis spectrophotometer, after persulfate oxidation to phosphate 17, using the ascorbic acid method.18
Trophic State Index (TSI)
To calculate the TSI values, the Carlson and Cunha et al. indexes were compared in order to select one criterion.6-11
Carlson index considers the TSI average (TSIa) calculated from measurements of SDD, Chla, and TP (n=96). The equations to calculate each TSI are showing below (Equation 1-3):
Cunha index considers the TSI average (TSItsr) calculated from measurements of chlorophyll a (Chla), and total phosphorus (TP) (n=96). The equations to calculate each TSI are showing below (Equation 4-5):
Classifications of the water reservoirs according TSI values were done according to both criteria, Carlson and Cunha et al. 6,11,19,20
Phytoplankton counting and identification
Samples were collected into the first 50 cm of the water column, using borosilicate containers of 250 mL, none preserved, but transported to the laboratory in complete darkness and cooling, proceeding immediately to their counting and cyanobacteria identification.7
Counting of cells (x 104 cell.mL-1) was made in fresh samples using a haemocytometer Neubauer. A differentiated counting of cyanobacteria was developed.
Taxonomic identification of cyanobacteria was fulfilled with different criteria till species when it was possible.21-28 The list of species will be presented, highlighting the toxic species.
Qualitative analyses
During the sampling some qualitative analyses were taking into account, based on determinations of some key indicators or attributes of the reservoirs like water discoloration, presence of algal bloom and algal scum; odor changes, macrophytes presence, and toxic cyanobacteria species occurrence.19,29
Statistical analysis
Statistical analyses (mean, standard deviation, and relative error), all considered P˂0,05. These were conducted using R studio (v 1.1.456) Sigmaplot (v 11.0.0.77) and Origin Pro 9. To analyse the quantitative similarity between samples, attending to TSI average, a parametric analysis between samples were done with resemblance measure using software Primer 6, considering a S18 Kulczynski, creating a lower triangular resemblance matrix previous to the cluster analysis.
Pair samples test with two tailed of significance was conducted to compare TSI obtained by both methodologies. It was included a Pearson Correlation analysis.
Results and discussion
Chlorophyll a concentrations
According to the calibration curve was possible to adjust the results of chlorophyll a in vivo using the resulting curve equation (y= 0, 001 3 x + 0,001 7; R2=0,996 1) where y is A675 and x is the concentration (µgL-1) (figure 2). The correction factor was calculated for each sample from the ratio Chla f: Chla DO 675, resulting an average of 6,902 5 ± 0,000 1. The limits values of Chla (µgL-1) were analysed, ranged between 19, 28 to 127, 43 µgL-1.
Trophic status of reservoirs
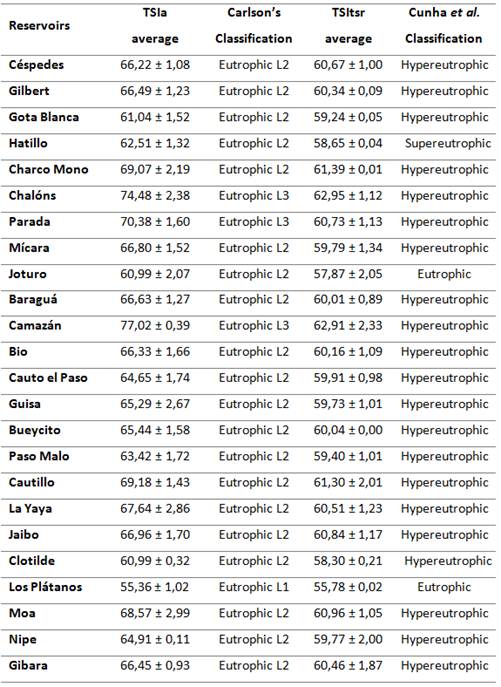
The results of TSI values of the twenty-four reservoirs studied are presented in table 1, as well as the status according to different classification criteria.6,11,19,20 Trophic State Index (TSI) is frequently used as biomass-related trophic state, and it is generally easy to understand, both in theory and practice, being useful for monitoring and management purposes, even punctually as a starting point for analysing data on water quality in the tropics/subtropics since this issue is of paramount importance worldwide.6 The TSI average (TSIa) is a better criterion to classify and characterize the trophic state of lakes and reservoirs than the use of separated index.20
During this study was confirmed the eutrophic status of all the reservoirs, which classified in different levels of eutrophication according to Cunha and Carlson criteria.6,11,19,20
Los Plátanos reservoir with TSIa = 55,36 ± 1,02, classified into the level 1 according to Carlson; but according to Cunha et al. (TSItsr =55,78 ± 0,02) the reservoir is in the limit of mesotrophy and eutrophyc. In this reservoirs highlight low levels of Chla. Los Plátanos is a small water reservoir, located near to El Salvador community (downstream), at north of Guantanamo city. The reservoir is near to a mountain system with forest coverage.
The highest values of both TSIs corresponds to Camazán (77,02 ± 0,39 and 62,91 ± 2,33) and Chalóns (74,48 ± 2,38 and 62,95 ± 1,12). Camazán reservoir receives direct run-off and contribution from agricultural areas, and the river received the influence of human pressure because of the settlements and Chalóns reservoir, (north of Santiago de Cuba city) receive the direct impact of Boniato town, agricultural areas, and cattle raising, impacting the water quality (table 1).
Even when this study evaluates TSI average calculated according to Carlson and Cunha, other authors use TSI calculated only with Chla with similar results. In this study, the TSI average was a better criterion to evaluate TSI in water reservoirs.20 These results need to be complemented with qualitative analysis, it was relevant to analyse the Mícara reservoir.
Table 1 Variation of TSI in twenty-four reservoirs and classification according to Carlson 19,20 and Cunha classification.6
According to Carlson, Mícara is considered into the eutrophic level 2, establishing as main attributes, the dominance of blue-green algae, algal scum probable, extensive macrophyte problems.11,19 Nevertheless in Mícara was detected algae bloom accompanied by water discoloration. It means that this reservoir have features of level 3 following Carlson criteria, but not correspond with attributes of eutrophic level 2.
All these analyses showed how is difficult to select one or other criteria to establish the eutrophic levels. Comparing media values of TSI obtained following Carlson and Cunha et al. equations 6,11,19,20, these are not significantly different (P<0,05) (59,80 ± 4,26 and 60,07 ± 1,44, respectively), but Carlson is the most used index. It was the main reason to analyse the data considering a cluster analysis of TSIa of Carlson classification.
The eutrophic conditions of water reservoirs are related to catchment uses, catchment populations linked with anthropogenic pressures, mainly because water polluted discharge, in those ecosystems resulting in inadequate management: A problem that needs to be solved.
Another important pressure is the aquaculture development, observed in all studied water reservoirs, due to fertilization practices which provide nutrients that condition the eutrophic status.
New criteria to classify the trophic status of studied reservoirs
Considering the possibility to adjust the classification attending to the specific environmental scenario and features of the reservoirs, a cluster analysis was conducted in order to group the reservoirs based on TSIa similarities, then the coincidence with TSIs ranges used in the Carlson classification was evaluated (figure 3). It allows considering at least four eutrophic levels but a re-assignation of TSIa values was necessary, as well as new attributes specification.

Fig. 3 Hierarchical cluster analysis of studied reservoirs based on TSIa. Proposal of five levels and new ranges in relation to limits of TSIa per group
As a result of this research, the Carlson criteria were modified, mainly in the eutrophic ranges (≥50). An extra level (L5) was proposed taking into account TSI values higher than 78 (the maximum TSI value detected during this study), was potentially possible.
Attributes
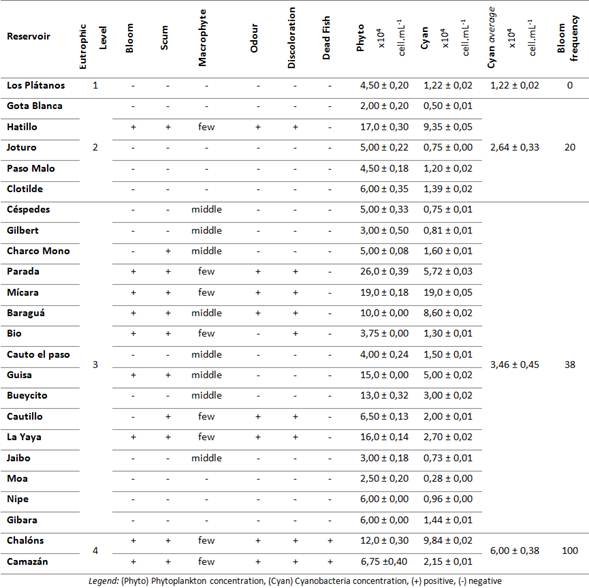
To establish the attributes, qualitative and quantitative indicators were taking into account to analyse the reservoirs attributes like phytoplankton and cyanobacteria counting, and some qualitative analyses like algal bloom and algal scum presence; as well as odour changes, macrophytes presence, and toxic cyanobacteria species occurrence were useful (table 2).
Cyanobacteria blooms were detected in 37 % of total reservoirs 9, these were distributed in level 2 (1 reservoir), level 3 (6 reservoirs) and level 4 (2) of eutrophy. In all of them toxic bloom-forming species were involved.
A bloom of toxic species: Cylindrospermopsis raciborskii and M. flos-aquae was identified in Hatillo (level 2).30,31 In this level 2, the bloom frequency was 20 %.
In the 16 reservoirs classified into the level 3, blooms were detected in six reservoirs (38 %), involving different toxic species: Microcystis aeruginosa (Parada and La Yaya), C. curvispora and Oscillatoria sp. (Baraguá), M. aeruginosa, M. flos-aquae, M. panniformis and M. wesenbergii (Mícara), Planktolyngbya limnetica and Oscillatoria sp. (Bio) and Synechococcus sp. (Guisa).
The bloom frequency in water reservoirs classified as level 4 was 100 %, being Synechocystis aquatilis (Chalóns) and C. raciborskii (Camazán) the species involved. Specifically, in these reservoirs, unpleasant odours, water discoloration, and dead fish were detected.
Scums were present in 46 % (11) of all the reservoirs, being the level 3 and 4 of eutrophication the most affected, mainly related to the bloom occurrence; highlighting the lower presence of macrophytes on these reservoirs (table 2).
According to these results, TSI can be easily used to predict short-term trophic dynamics of the studied reservoirs, even when not provide data concerning phytoplankton composition, but it is necessary to take into account. There are some researches that link the trophic status of reservoirs with cyanobacteria blooms, which support these results.32
Phytoplankton
Phytoplankton cell density (table 2) ranged from 2,00 ± 0,20 x 104 cell.mL-1 (Gota Blanca) to 26,0 ± 0,39 x 104 cell.mL-1 (Parada). The average values have an increasing tendency in relation to trophic level, being 6,9 ± 0,52 x 104 cell.mL-1 in level 2, 8,98 ± 0,67 x 104 cell.mL-1 in level 3 and 9,37 ± 0,26 x 104 cell.mL-1 in level 4. The reservoirs with the higher density of phytoplankton cells were Parada, Mícara, Hatillo, la Yaya, Guisa and Chalóns (table 2). The phytoplankton concentration was in the range of previous reports in studies conducted in tropical reservoirs, with high levels of cyanobacteria and cyanotoxins 33,34
Cyanobacterial concentrations (table 2) ranged from 0,50 ± 0,01 x 104 cell.mL-1 to 9,84 ± 0,02 x 104 cell.mL-1, being the highest average concentrations those corresponding to level 4 (6 ± 0,38 x 104 cells.mL-1), which was significant different with the rest of levels. Concentrations of cyanobacteria range from 1,22 ± 0,02 x 104 cells.mL-1, even though, there are no significant variation between concentration of cyanobacteria between levels 2 and 3 (2,64 ± 0,33 and 3,46 ± 0,45 x 104 cells.mL-1, respectively), there is a significant differences in relation with level 1. There is a correlation between eutrophication level and the concentration of cyanobacteria.
The results suggest that the risk because of cyanobacteria occurrence is closely related to the eutrophic level, being imminent in reservoirs classified in level 4 (table 2). High loads of nutrients and other factors can condition the eutrophication and collaterally the growth of potentially toxic cyanobacteria blooms is detected. The mechanisms underlying cyanobacterial growth and bloom-forming need further research. A general analysis allows to propose a new trophic state classification, with TSI values and attributes modified according to the results of the cluster analysis of the study reservoirs, it has consistent differences in relations with Carlson classification (table 3).
Table 3 Proposal of trophic status classification of eutrophic water reservoir at the eastern of Cuba considering range, trophic levels, and attributes
| TSI values | Trophic status | Attributes |
| <58 | Eutrophic (level 1) | Lower boundary of classical eutrophy: decreased transparency, abundance of cyanobacteria, low level of total phosphorus, good for fisheries, acceptable to water supply. |
| 58-64 | Eutrophic (level 2) | Decreased transparency, cyanobacteria could be dominant, algal scum or algal bloom probable, extensive macrophyte problems, low risk of toxic cyanobacteria. Acceptable for fisheries and water supply. |
| >64-73 | Eutrophic (level 3) | Dominance of cyanobacteria, algal scum probable, heavy algal bloom probable. Extensive macrophyte problems. Medium to high risk of toxic cyanobacteria. Limited quality for fisheries and water supply. |
| >73-78 | Eutrophic (level 4) | Heavy algal blooms, algal scum, fish kills, few macrophytes. High risk of toxic cyanobacteria. Restrictions for fisheries and water supply. |
| >78 | Eutrophic (level 5) | Higher boundary of eutrophy. Persistent bloom of toxic cyanobacteria, fish kills, bad odours, bacterial growth. Fisheries and water supply prohibited. |
Cyanobacteria occurrence
In all the studied reservoirs were identified 38 species of cyanobacteria. The reservoirs with higher cyanobacteria diversity were Baraguá (20), Bio (16) and Camazán (16) (table 2).
Toxic species represent the 61 % of total cyanobacteria, highlighting the occurrence of toxic and bloom-forming species, which are usually involved in episodes of animal and human toxicity worldwide (Microcystis sp., Cylindrospermopsis sp., Planktolyngbya sp., Oscillatoria sp., Synechococcus sp. and Synechocystis sp.).30,35-37
The most frequent species were Synechocystis aquatilis and Aphanocapsa sp. with a spacial frequency of 62, 5 %, followed by Planktothrix sp., Merismopedia punctata (54 %) and Romeria simplex (50 %). Microcystis genus was the better represented with six species which are present in 70 % of the reservoirs
Concerning toxic species diversity was higher in Baragua reservoir (13 species), which represents 65 % of the total cyanobacteria species in this reservoir; followed by Bio, Cautillo, and Camazán which have 10 toxic cyanobacteria species, representing a 62, 67 and 62 %, respectively. It is an important rick because of the potential productions of cyanotoxins (table 4).
Table 4 Distribution of cyanobacteria species in eutrophic levels and studied reservoirs
| Eutrophic Level | 1 | 2 | 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reservoir | Los plátanos | Gota Blanca | Hatillo | Joturo | Paso Malo | Clotilde | Céspedes | Gilbert | Charco Mono | Parada | Mícara | Baraguá |
| Cyanobacteria species | ||||||||||||
| x | x | |||||||||||
| x | x | x | ||||||||||
|
|
x | x | x | x | x | x | x | x | ||||
| x | x* | |||||||||||
| x* | x | |||||||||||
|
|
x | x | x | |||||||||
|
|
x | |||||||||||
|
|
x | |||||||||||
| x | x | x | x | x | x | x | ||||||
| x* | x* | |||||||||||
| x | x | |||||||||||
| x* | x* | |||||||||||
| x | x | x* | x | |||||||||
| x | x | x | x | x | ||||||||
| x | x* | |||||||||||
|
|
x | x | x | x* | ||||||||
| x | x | |||||||||||
|
|
x | x | x | x | ||||||||
|
|
x | x | x | x | x | |||||||
|
|
x | |||||||||||
|
|
x | |||||||||||
| x | x | x | x | x | x | x | ||||||
|
|
x | |||||||||||
| x | x | x | x | x | x | |||||||
| x | x | |||||||||||
|
|
x | x | x | x | x | x | x | |||||
| x | x | x | ||||||||||
| x | x | |||||||||||
|
|
x | |||||||||||
|
|
x | x | ||||||||||
|
|
x | x | x | |||||||||
| x | x | x | x | x | x | |||||||
| x | x | x* | x | x | ||||||||
| x | x | |||||||||||
| x | x | x | x | x | ||||||||
| x | x | |||||||||||
| x | x | x | x | |||||||||
| x | x | x | x | |||||||||
|
|
x* | x | x | |||||||||
| x* | x | x | ||||||||||
|
|
x | |||||||||||
|
|
x | x | x | x | x | x | x | x | ||||
|
|
x | x | x | x | x | |||||||
|
|
x* | x | x | x | ||||||||
| x | x | x | x | x | x | x* | x | |||||
|
|
||||||||||||
Legend:*= Bloom
Conclusions
This study allowed to demonstrated that TSIa is an important tool to predict the occurrence of toxic cyanobacteria, specifically at the eastern of Cuba where it is an important risk management issues. All the reservoirs were classified as eutrophic but considering different levels and all of them were affected by toxic cyanobacteria blooms, confirming the high risk of these water reservoirs. Integral management needs to be considered in order to comply with safe water quality standards. In this context, the classification proposal can be useful to the water reservoir management at the eastern of Cuba