Introduction
Within the mining industry there is a wide variety of processes and technological treatment alternatives, and each one separately are the focus of environmental pollution by lock of treatment if its waste is not managed in an adequate, responsible and conscientious manner. These anthropogenic activities accounts for redistribution of several toxics and heavy metals into the environment.1
Today the most commonly used reagent for the gold leaching process is cyanide due to its high ore recovery, robustness and relatively low costs. However, a number of environmental damages occur very often at various gold mines around the world due to poor management of these wastewaters at the end of the process, which has raised concerns about the use of cyanide as a leaching reagent.2 When cyanide comes into contact with dissolved metal ions, metal-cyanide complexes are formed. Depending on the metal involved, these complexes may be weak acid dissociable (WAD) as is the case of Ag, Cd, Cu, Ni, Zn, Hg or strong acid dissociable (SAD) such as Fe, Au, Co, Pt or Pd complexes.3 Heavy metals are of great concern to human health particularly because of their ability to bio-magnify, bio-accumulate and persistence in the environment.4-6
Many countries after serious accidents involving the use of cyanide in the gold mining process have conducted promising research to find efficient and less toxic technological alternatives. Several studies revealed that thiosulfate leaching can be an efficient and less dangerous process for gold recovery and should be seen as a replacement for cyanide in decades ahead.7
The conventional processes for wastewater treatment in gold mining industry are complex and costly, due to the high concentration of compound and high volume of wastewater subject studies. On the other hand, must be consider the cost of disposal of effluent, geographical location, climatic conditions and government restrictions.7 In this sense, many environmental organizations has called to pay attention about the quality of life of the people that live around the processing plant.
For these purposes, different methods have been developed, most of which involve chemical oxidation such as alkaline chlorination, oxidation with copper-catalyzed hydrogen peroxide, ozonization, the use of ultraviolet radiation, TiO2 photocatalysis, adsorption on metal-impregnated activated carbon, biological degradation, etc.8-12 With these methods, it is possible to produce a cyanide free effluent, but frequently the following problems appear: a very expensive treatment caused by the high consumption of reagents or a too long time of treatment.13
The possibility of oxidizing cyanide with ozone is being studied since 1959. Ozone gas is one of the most powerful oxidizers available. In industrial basis, ozone has been used with good results in spent developing baths, in electroplating wastes and in cyanidation effluents.14-16 It is a promising chemical reagent to treat cyanide effluents because it presents many advantages: no need of transportation, storage and handling of chemicals, a very rapid and complete decomposition of cyanides, low maintenance, low labor requirements and simple operation, among others.17
The study of wetlands as ecologically and alternative treatment methods has shown that water and waste water are “naturally purified” by biological organisms and other physic-chemical mechanism that "consume" pollutants and also seeps through rock formations and porous materials in the soil.18 In artificial or constructed wetlands, the aim is to simulate or reproduce the characteristics and conditions of a natural wetland in a strategic location where there is a discharge of contaminated water from a process. The plants are known to absorb and accumulate metals in their organs, thereby reducing the concentration and risk of the metals in the wastewater.19-21
In Cuba, the cyanide salt leaching process is regularly and extended used method of obtaining gold due to its high recovery efficiency and its low production cost. However, its main disadvantage is the generation of significant volumes of wastewaters, with a high concentration of cyanide and heavy metal species.22
The fundamental problem gave rise to this work is associated with a series of technological and operational irregularities carried out at a gold ore processing plant. These situations have led to the generation and unwanted accumulation of a significant amount of toxic wastewater (approximately 56,000 m3), with a high cyanide and heavy metal species concentration. These aqueous pollutants are currently temporarily stored in containment pools and natural lagoons, close to reaching their maximum capacity limit.
Several environmental studies carried out at the plant UEB “Gold Ores Processing”, belonging to Geominera Centro Enterprises, and to the Geominsal Group of the Cuban Ministry of Industry and Mines, located in Placetas town, Villa Clara, central cuban province, had demonstrated that this waste water compound composition exceeded standards spilled by Cuban normative NC 27: 2012.23
This situation is extremely risky, since in the event of possible meteorological phenomena join by heavy rainfall, which is frequent in our country, it represents a potential damage of contamination for the inhabitant living around the pollution focus and to the environment, which requires the urgent planning of actions for its management to guarantee a final solution to these wastewater according with current legislation, being compatible with the environment and sustainable development.
The main objective of this researching work is to evaluate from the technical, economic and environmental point of view, two technological alternatives for the management of wastewater from this gold ores processing plant.
Materials and methods
Analytical methods used in the characterization of wastewater
The sampling for analytical studies was carried out in the three reservoirs of the processing plant: emergency lagoons A and B (EL-A, EL-B) and tailings dam (T.D.), (see figure 1), on the basis of the criteria of taking specific samples, according to the regulations of the National Institute of Hydraulic Resources.23 The following parameters were determined: pH, conductivity, free cyanides, WAD and total cyanides, chlorides, residual chlorine and heavy metals, according to the Standard Methods for Examination of Waters and Waste Waters in APHA,24 (see table 1). Cyanide was analyzed by titration with silver nitrate (Standard Methods 4500-CN-D), spectrophotometrically (WAD), and with the help of a cyanide-specific electrode (Standard Methods 4500-CN-F). Metals were analyzed by atomic absorption spectrometry (AAS), using a PYE UNICAM spectrophotometer model SP 9.
The equipment and glassware used in all the determinations are duly verified by the Territorial Standardization Office (TSO). All the reactive employed in the analytical test were analytical grade (PPA) commercialized by Merck, Reachim and BDH companies.
Table 1 Quality parameters and analytical methods used in wastewater characterization
| Parameters | Analytical Method used /24/. Equipment |
| WAD cyanide (mg/L) | Spectrophotometric method (4500-CN, I). Genesis Spectrophotometer |
| Total cyanides (mg/L) | Acid distillation and titrimetric method (4500-CN, C+D) |
| Free cyanides (mg/L) | Titrimetric method with silver nitrate (4500-CN, D) Cyanide-Selective Electrode Method (4500-CN, F) |
| Heavy Metals (mg/L) | Atomic Absorption Spectrometry (PG-990, PG-Instruments) |
| pH ( U) | Potentiometric method. WTW pHmeter. |
| Conductivity (μS/cm) | Potentiometric method. WTW conductivity meter |
Results and discussion
Physical-chemical characterization of wastewater
The physical-chemical characterization of the wastewaters discharged into the emergency lagoons A and B and the tailings dam was carried out for one year determining that, in all cases, the concentration of cyanides exceed the Cuban Standard Normative NC 27: 2012,25 Also were detected heavy metals, and a certain alkalinity level, which indicates that these waters must be treated before being disposed or reused (table 2).
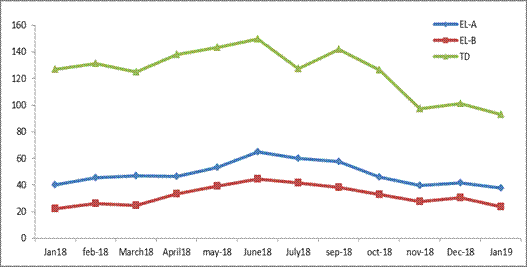
Figure 2 graphically shows the variation of the average concentration of total cyanides over time in the two lagoons and the tailings dam from January 2018 to January 2019. These high cyanide values (22-150 mg/L) are caused by the low efficiency in the oxidation stage and low mass transfer in the aeration process of the technology used to date in the plant (alkaline chlorination). These treatment stages for cyanides elimination are not effective, and represent an inefficient method for the removal of complex ferrocyanides considering others methods applied in the world.
To propose a treatment, should be a particular attention to nickels concentration in the tailings dam (TD), which averaged between 800 and 900 mg/L. On the other hand, the pH of the water in the three ponds has always remained in the alkaline range, which is favorable for maintaining the stability of free cyanides in solution (table 3).
Table 2 Heavy metals concentration (mg / L) in the containment reservoirs (average values).
| Metals | Zn | Cu | Co | Pb | Mn | Cd | Fe | Cr | As | Ni |
| EL-A | 0.13 | 1.1 | 1.5 | < 0.1 | < 0.05 | < 0.05 | < 0.05 | < 0.1 | 2.3 | 24.76 |
| EL-B | 0.14 | 2.6 | 1.75 | < 0.1 | < 0.05 | < 0.05 | < 0.05 | < 0.1 | 1.5 | 31.78 |
| TD | 0.24 | 3 | 2.1 | < 0.1 | < 0.05 | < 0.05 | < 0.05 | < 0.1 | 6.4 | 854 |
Other parameters, observed regularly in the Tailing Dam are: the electrical conductivity and chloride concentration. These values varied between 2500 - 3000 (µS/cm), and between 50 and 300 (mg/L) respectively.
Table 3 pH variation in the EL-A, EL-B and TD since January 2018 to January 2019.
| pH | Jan18 | Feb18 | March18 | April18 | May18 | June18 | July18 | Sep18 | Oct18 | Nov18 | Dec18 | Jan19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EL-A | 8.7 | 8.5 | 8.2 | 8.3 | 8.6 | 8.5 | 8.7 | 8.1 | 8.0 | 8.3 | 8.2 | 8.5 |
| EL-B | 8.6 | 8.4 | 8.1 | 8.1 | 8.5 | 8.3 | 8.6 | 8.0 | 7.8 | 8.1 | 8.4 | 8.2 |
| TD | 9.6 | 9.7 | 9.5 | 9.5 | 9.4 | 9.6 | 9.6 | 9.3 | 9.3 | 9.4 | 9.5 | 9.5 |
Technological alternatives
Two technological alternatives were proposed and analyzed for the treatment of the wastewater currently stored in the containment lagoons.
The first was suggested and implemented by specialists and technicians of a gold ores processing plant (Alternative # 1), and proposes to add a solution of sodium hypochlorite in alkaline medium (pH: 10-12) to achieve the oxidation of cyanides, a well-known method named “alkaline chlorination”. This alternative has nine technological stages (figure 3) and its main advantages and disadvantages are shown in table 4.
As a second option, an alternative, valued by CEQA´s researchers, belonging to the Faculty of Chemistry and Pharmacy of the Central University “Marta Abreu” of Las Villas (Alternative # 2), is incorporated, proposing the use of an advanced oxidation stage with ozone gas, addition of ferric chloride, and the incorporation of an horizontal subsurface flow constructed wetland (HSSFW) at the end of the process as a tertiary treatment.
The estimated treatment capacity of the plant is 15 to 20 m3/h, 180 to 240 m3/day and 63 000 to 84 000 m3/year.
Alternative # 1
A wastewaters treatment consisting of nine stages is proposed:
Table 4 Advantages and Disadvantages of the alkaline chlorination (Alternative # 1)
| Alternative 1 | ||
|---|---|---|
| Advantages | Disadvantages | |
| The main sources of supply for the reagents are domestic, at acceptable costs and near to the plant. Sodium hypochlorite has demonstrated versatility in the elimination free cyanide and in turn hydrolyzing nickel and copper. Simple technology, using atmospheric pressure, and semi-continuous flow. Natural and domestically produced filtering materials (zeolites) are used, which can function as ionic collectors, and activated carbon from Baracoa as adsorbent, although this has a higher price. | High number of treatment steps, including two recirculation circuits. High consumption of chemical reagents. The efficiency in the cyanides oxidation with air and the mass transfer in the aeration process is low. The elimination of all cyanides (free and WAD only) is not guaranteed. Adequate mixing is not ensured in the arsenic precipitation stages and in the addition of flocculants and coagulants. | |
Alternative # 2. (Cyanide’s Oxidation with Ozone)
The technological variant proposed by the authors employ the treatment process equipment actually in operation, with the principle of using the maximum technological resources available, but with a simpler technological scheme, incorporating an advanced ozone oxidation method and a tertiary treatment with wetlands. This alternative has six technological stages (figure 4) and its main advantages and disadvantages are shown in table 5.
Table 5 Advantages and disadvantages Alternative # 2
| Alternative 2 | ||
|---|---|---|
| Advantages | Disadvantages | |
| Existing processing plant equipment and pumping systems will be used. Less number of stages and less water consumption. The use of chemical reagents and the generation of metal sludge are minimized. High efficiency to removal cyanides species (CN-, CN- (WAD) and CN- (COMPLEX)). Possible reuse of treated water (higher quality), after treatment. | An industrial ozone generator needs to be purchased (initial investment). | |
The comparison of the two alternatives proposed for the elimination of the main pollutants contents in the wastewater of the lagoons, shows that the alternative # 2, based on advanced oxidation with ozone in alkaline medium, is the most appropriate at the present time. This technological variant, presents the greatest advantages from the operational point of view, technological design, efficiency in the elimination of total cyanides (+98%), minimizing the consumption of process water, and chemical reagents. On the other hand, the negative impacts that could be generated on the environment and inhabitants living near the treatment plant are minimized. Figure 5 shows the flow diagram of technological process for the second alternative.
Economic analysis of the proposed alternatives
The technical-economic analysis of a technological process is development to determine if the projected investment is capable of satisfying the requirements that have originated it and if it is economically feasible, objectively evaluating the results and evaluating the different possible alternatives (table 6).
Table 6 Summary costs of the proposed alternatives
| Costs | Alternative # 1 | Alternative # 2 |
|---|---|---|
| Investment cost ($/year) | 7 479 | 18 467 |
| Water consumption cost ($/year) | 2 695 | 396 |
| Cost of equipment ($/year) | 4 922 | 12 153 |
| Reagent cost ($/year) | 633 924 | 44 387 |
| Operational costs ($/year) | 11 877 | 11 877 |
| Costs of energy consumption ($/year) | 4 739 | 5 305 |
| Total cost of production ($/year) | 705 725 | 70 947 |
| Unitary cost ($ / m3 of treated wastewater) | 29.65 | 2.9 |
One of the most important elements in these analyses of the economic effectiveness of the alternatives is what is known as the "investment cost", which in financial or economic terms constitutes the total expenditure on human and material resources necessary for the construction and start-up of the new production capacities.
For the calculation of the different costs associated with each technological alternative, was used the methodology proposed by Peters.25 This design facilitates multiple factor analyses, quickly and effectively, allowing for assessments or adjustments in each case, for decision making.
Considering the results, the high production costs of Alternative # 1, are associated mainly with the high water and chemicals reagent consumption, which significantly increase the total cost of treatment. The second alternative requires an initial investment, incorporating to the process an ozone generator. This option simplifies the process technology and minimizes the chemicals and water consumption, achieving a lower unitary cost of treatment according to the international standards for these types of technologies. This is the fundamental criterion to be considered for its practical implementation in the gold ores processing plant.
Dynamic analysis of the cost. NPV, IRR, and RPD calculation
The technical-economic analysis of any activity is a complex studies of each of the links constitute and contribute to the economic-productive activity, being one of the functions of the organization and scientific direction of the production. It allows it to determine if the projected investment satisfice the requirements, objectively evaluating later the results.
An assessment of the feasibility of the investment was made on the basis of the calculation of the dynamic indicators NPV (net present value), IRR (internal rate of return) and RPD (payback period), taking an interest rate of 15 %. The results obtained are summarized in table 7.
Table 7 Values of the feasibility indicators of the variant under study (alternative #1).
| Indicators | Values |
|---|---|
| Net present value NPV [$] | 175 761 |
| Internal rate of return. IRR [%] | 98 |
| Recovery period discounted. RPD [months] | 12 |
The values reflected in this table indicate the feasibility of the proposed technology as the NPV is greater than zero and the recovery is carried out within a period of 12 months (one year).
Conclusions
The chemical characterization of the wastewater from the gold ores processing plant confirms the existence of high levels of toxic substances and heavy metals, which give the plant its highly hazardous and toxic characteristics, representing a potential threat to the surrounding population, the health of workers, living organisms and the environment. Investment, production and total costs were estimated for both alternatives, highlighting the high production costs of Alternative # 1, associated mainly with the great consumption of chemicals and process water. The technology proposed by CEQA´s researchers (cyanide’s advanced oxidation with ozone) requires an initial investment for the acquisition of an ozone generator, but it simplifies the process and minimizes the consumption of chemicals and process water, achieving a unit cost of treatment in accordance with international standards for these technologies, making it the most appropriate for its implementation. The economic indicators values shows that, Alternative # 2, is technically and economically viable, and could be quickly implemented, since it has a NPV of $ 175,761 and the initial investments could be recovered in approximately 12 months