Introduction
The increased use of traditional medicines has led to growing dedication on research and development focused toward such preparations.1 A knowledge of the chemical constituents of plants is desirable not only for the discovery of therapeutic agents, but also because such information may be of value in disclosing new sources of such economic materials as tannins, gums and precursors for the synthesis of complex chemical substances. In addition, the knowledge of secondary metabolites of plants would further be appreciated in determining the actual value of traditional remedies.2
Boophone disticha is a common bulb in Zimbabwe because of its various uses.3 Its taxonomy is the following: Order: Asparagales, Family: Amaryllidaceae, Subfamily: Amaryllidoideae, Genus: Boophone, Species: B. Disticha.
Is used to treat various ailments such as rashes and skin disorders including eczema. Rheumatic pains, arthritic swelling, sprains, muscular strains, painful wounds, eye conditions, headaches, anxiety, the pain of abrasions and inflammatory conditions have also been treated using its plant parts. The inner fresh scales are applied to burns and the outer dried scales of the bulb are used as an outer dressing for circumcision.4,5 Bulb decoctions are administered either orally or as enemas to adults suffering from headaches, abdominal pain, weakness, sharp chest pains and persistent bladder pains. Treatment of gastrointestinal problems such as varicose ulcers and relief of urticarial as well as treatments for cancer are also part for its therapeutic uses.4,5
An assemblage of alkaloids (mostly isoquinolines) are responsible for most of the effects of Boophone disticha and these include buphanamine, buphanidrine, buphanine buphanisine, haemanthamine, nerbowdine, undulatine, lycorine, crinamidine, crinine, 3-O-acetylnerbowdine, ambelline, buphacetine and distchamine.6,7,8 Some compounds have also been isolated from a volatile oil, containing furfuraldehyde, acetovanillone, chelidonic acid, copper, laevulose, pentatriacontane, ipuranol and a mixture of fatty acids.3,9
Boophone disticha has very wide ethno pharmacological uses and has common usage in Zimbabwe and the southern African countries.8 The plant is highly toxic when taken orally and it has grown in popularity for its toxicity than for its therapeutic value. Little or no research has been done to develop the topical use of Boophone disticha. Given the therapeutic properties of Boophone disticha, what more information can we find from study of metabolites present in this plant?
Several previous phytochemical investigations done using this medicinal bulb has reported various phytoconstituents with alkaloids being the most studied secondary metabolites but however there is no report documenting the screening of bioactive principles using successive extracts of the bulb and roots, using solvents of varying polarity. The individual phytochemical compounds of B. disticha roots have never been screened before. This part of the plant is poorly described in the literature. Nair and Van Staden 10 cites that roots are burned, ground and the powder applied to the area experiencing paralysis. It is known that alkaloids are related with neurologic drugs.
All the previous explanations carry us to the following aim: To qualitatively compare secondary metabolites in roots and fresh inner layers of Boophone disticha bulb, especially alkaloids through IR spectroscopy. This technique may help to find some functional groups presents in alkaloids and allow the comparison with previous reported compounds.6,9
Methods and materials
The plants were collected some 20 km from Bindura, the provincial capital of Mashonaland Central Province of Zimbabwe in month of March 2018 at 8.00am, with 28ºC (summer in south hemisphere). The leaves and roots were removed from the bulbs. The dry papery layers were removed and some fresh bulbs were peeled and left in the shade to dry. The other fresh bulb was peeled and cut into very small pieces which were then immediately used for extraction. Figure 1 shows the plant Boophone disticha.
Maceration
Fresh bulb layers were peeled and cut into very small pieces and 250g of this plant material was placed into a 1000ml amber bottle. 500ml diethyl-ether was added and the mixture was left for 72h at room temperature. Roots were nicely cleaned to remove all the soils and dirty without use of water. The roots were then cut into small pieces and 50g portion was mixed with 200ml Diethyl-ether in a 500ml amber bottle and left for 72h. The two container bottles were shaken at intervals during the 72h period. After the maceration for 72h the solvent was decanted into respective labelled containers which were tightly closed and then stored at room temperature. 500ml ethanol was added to the bulb residues and 200ml ethanol to the roots residues and both mixtures were left to stand for 72h. The solvents were decanted and the procedure was repeated with water. Maceration was used in order to preserve thermo labile metabolites if they were present while it had already been used by Botha and collaborators.4 The successive extraction method was used as was done as describe the bibliograhy.11 Phytochemical analysis was done using standard procedures with minor modifications as used by Bokhad and collaborators (2012).12 Other literature for extraction and phytochemical screening were consulted too.13
Fractionation of extracts
This method was developed by this research team. Extract fractionation method consist in that each of the principal extract is divided into three fractions (with the third fraction being of the same solvent as the principal extract). The solvent of each fraction is evaporated in water bath. The residual was dissolved in a new solvent. For the three main extract the fractionation was the following: Diethyl ether extract was divided into hexane, toluene and diethyl ether fractions. The ethanol principal extract was divided into dichloromethane, ethyl acetate and ethanol fractions. The water extract was divided into acetonitrile, 1:1 acetonitrile-water and water fractions. This method is good for separation of complex crude extracts as it helps in further separating metabolites based on their polarity.It is useful in obtaining pure secondary metabolites byeither dissolving impurities into a certain solvent which does not dissolve the wanted phytochemical compound(s). The compounds wanted may in another case be selectively dissolved in a certain solvent based on its polarity.
Alkaloid extraction
The alkaloid extraction process is based on the procedure developed by the Research Group Bioactive Compound and Green Chemistry from University of Oriente, Cuba.14 Also, some papers were consulted for alkaloid extraction.15,16
Successive extraction used in this study with fractionation were meant to provide a more detailed secondary profile of the bulb inner layers and roots which would be easier to compare. The extraction of the alkaloids was also done using the acid-base method a commonly used method. The powders used in the extraction were also analysed in their crude form using FT-IR technique. FT-IR spectra were acquired on a JASCO 6200 equipment with single-reflection ATR accessory. All the solvents were pure for synthesis and the metabolite test were prepared in the laboratories of Pharmacy Department, University of Oriente.
Results and discusion
Many metabolites tested positive in the various extracts and these include alkaloids, coumarins, phenolics & tannins and steroids (table 1). The strong positive of the alkaloids in Boophone disticha was expected from literature.17
Table 1 Preliminary screening of the principal extracts
| Metabolites | Test/Solvent | Diethyleter | Ethanol | Water | |||
| Roots | Bulb | Roots | Bulb | Roots | Bulb | ||
| Alkaloids | Mayer’s reagent | ++ | ++ | +++ | +++ | ++ | ++ |
| Wagner’s reagent | +++ | +++ | +++ | ++ | + | +++ | |
| Flavonoids | Test for flavones | - | - | + | ++ | - | - |
| Test for Coumarins | +++ | +++ | +++ | ++ | ++ | ++ | |
| Phenolics and Tannins | Lead acetate | + | ++ | +++ | +++ | +++ | +++ |
| Gelatine test | ++ | ++ | + | + | ++ | + | |
| Saponins | Foam test | - | - | - | - | - | - |
| Steroids | Liebermann-Buchard’s test | - | + | + | ++ | + | + |
| Salkowski’s test | + | ++ | +++ | +++ | ++ | + | |
| Quinones | Borntrager’s test | - | ++ | + | - | +++ | + |
Key: +++ strongly positive, ++ moderate positive, + positive, - negative.
The table 2 shows strong presence of alkaloids and coumarins in diethyl ether and ethanol extracts for both roots and bulb. Ethanol and water extracts strong positive results for the lead acetate test for phenolics and tannins while steroids were strongly present in ethanol extract. Quinones had a positive result in diethyl ether bulb extract and water roots extract. The ethanolic extracts for both roots and bulbs had relatively more secondary metabolites testing positive.
Table 2 Preliminary screening of fractionated diethyl ether extracts.
| Metabolites | Test/Solvent | n-hexane | Toluene | Diethylether | |||
| Roots | Bulb | Roots | Bulb | Roots | Bulb | ||
| Alkaloids | Mayer’s reagent | + | + | - | - | + | ++ |
| Wagner’s reagent | +++ | +++ | - | - | ++ | ++ | |
| Flavonoids | Test for flavones | - | - | + | - | - | - |
| Test for Coumarins | + | - | + | - | ++ | +++ | |
| Phenolics and Tannins | Lead acetate | + | + | + | + | - | + |
| Gelatine test | ++ | ++ | +++ | ++ | + | ++ | |
| Saponins | Foam test | - | - | - | - | - | - |
| Steroids | Liebermann-Buchard’s test | - | - | - | + | - | + |
| Salkowski’s test | + | + | + | ++ | + | ++ | |
| Quinones | Borntrager’s test | + | + | ++ | ++ | +++ | + |
In the table 2 phenolics and tannins have generally positive results for gelatin tests for all extracts. Alkaloids tested negative in all toluene extracts. The Wagner’s test had better positive results for alkaloids than the Mayer’s test. Coumarins were again positive for the diethyl ether extracts. The root diethyl ether extract had a strong positive result for quinines compare to toluene which had a moderately positive result.
Generally, dichloromethane had few positive results compared to the ethyl acetate and ethanol extracts (table 3). The dichloromethane extracts for the roots had a negative result for the Mayer’s reagent test while only the Wagner and gelatine tests had positive results. The results of the ethanol re-extracts were similar to those of the principal ethanol extract.
Table 3 Preliminary screening of fractionated ethanol extract
| Metabolites | Test/Solvent | Diclhoromethane | Ethyl Acetate | Ethanol | ||||
| Roots | Bulb | Roots | Bulb | Roots | Bulb | |||
| Alkaloids | Mayer’s reagent | - | + | +++ | +++ | ++ | +++ | |
| Wagner’s reagent | ++ | ++ | +++ | +++ | +++ | ++ | ||
| Flavonoids | Test for flavones | - | - | + | ++ | - | - | |
| Test for Coumarins | - | + | - | + | + | ++ | ||
| Phenolics and Tannins | Lead acetate | - | + | ++ | ++ | +++ | ++ | |
| Gelatine test | ++ | ++ | ++ | + | + | + | ||
| Saponins | Foam test | - | - | - | - | - | - | |
| Steroids | Liebermann-Buchard’s test | - | + | + | ++ | + | + | |
| Salkowski’s test | - | ++ | + | + | +++ | ++ | ||
| Quinones | Borntrager’s test | - | ++ | + | - | ++ | - | |
Acetonitrile like dichloromethane had few positive results when compared to the other re-extracts and its root extract had also a negative test to Mayer’s reagent test (table 4). The 1:1 mixture of acetonitrile and water had almost similar results to the water re-extract with the difference being a negative for the bulb extract.
Table 4 Preliminary screening of fractionated water extracts
| Metabolites | Test/Solvent | Acetonitrile | Acetonitrile:Water | Water | ||||
| Roots | Bulb | Roots | Bulb | Roots | Bulb | |||
| Alkaloids | Mayer’s reagent | - | + | + | ++ | +++ | ++ | |
| Wagner’s reagent | + | + | ++ | +++ | +++ | +++ | ||
| Flavonoids | Test for flavones | - | - | + | ++ | - | - | |
| Test for Coumarins | - | + | ++ | ++ | ++ | + | ||
| Phenolics and Tannins | Lead acetate | - | - | + | + | ++ | ++ | |
| Gelatine test | ++ | ++ | ++ | + | - | ++ | ||
| Saponins | Foam test | - | - | - | - | - | - | |
| Steroids | Liebermann-Buchard’s test | - | + | + | ++ | + | + | |
| Salkowski’s test | + | ++ | +++ | +++ | ++ | + | ||
| Quinones | Borntrager’s test | - | ++ | + | - | +++ | + | |
It is relevant the strong presence of alkaloids in the principal extracts which are consistent with previous studies where water and ethanol were widely used for extraction of alkaloids or generally in screening. The roots and bulb had similar results for diethyl ether, ethanol and water, principal extracts and re-extracts which can be a result of similar alkaloidal constituents. Literature has some screening showing similar activity like in the antimicrobial screening done by Cheeseman which can also be partly explained with these results.17 Toluene had negative results, while acetonitrile also had relatively low positives for both bulb and roots.
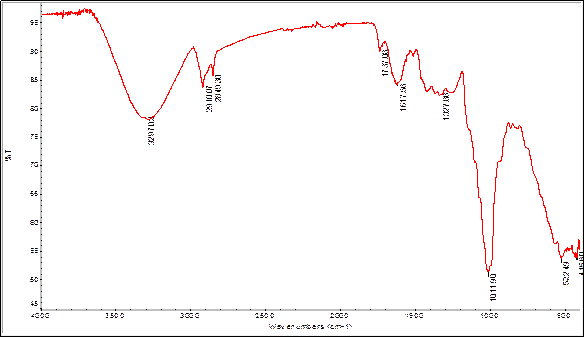
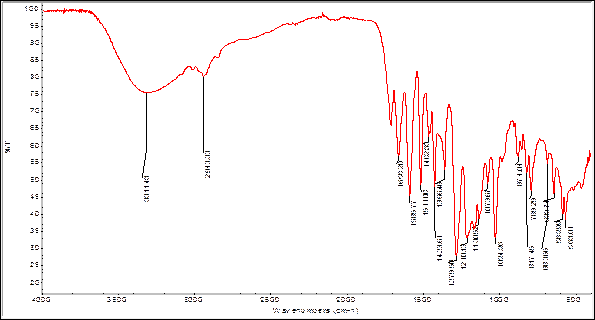
In order to find some signals of relevant functional groups indicating the presence of some metabolites IR spectra were obtained for bulb and root powders, as well as alkaloids extracts. The figures 2 and 3 show the IR spectra of Boophone disticha bulb powder and extracted alkaloids from bulbs.
The IR spectra of bulb powder and extracted alkaloids crude from bulbs, show differences as well as many similarities, indicating that the extracted compounds are present in the powder (tables 5 and 6).
Table 5 Characteristic IR-bands of Boophone disticha bulb powder.18
| Frequency (cm-1) | Assignation |
|---|---|
| 3297 | ν O-H carboxylic acids or alcohols |
| 2910 | ν C-H Csp3 |
| 2849 | ν C-H Csp3 |
| 1737 | ν C=O carboxylic acids |
| 1617 | ν C---C Phenyl ring |
| 1327 | C-O carboxylic acids, ν C-N |
| 1011 | ν C-O alcohol, ν C-N |
Note: stretch vibration (ν), bend vibration (δ in the plane, γ out of plane).
Alkaloids that phytochemical tests result positive in many solvents (see tables 1-4), contains C-N bond in the structure. This vibration appears between 1400 and 1000cm-1 as many bands with medium intensity, even though this region in complicated due to the coexistence of several other vibrations like ν C-O of alcohols and carboxylic acids.18
Table 6 Characteristic IR-bands B. disticha extracted alkaloids from bulbs.18
| Frequency (cm-1) | Assignation |
|---|---|
| 3311 | ν O-H carboxylic acids or alcohols |
| 2943 | ν C-H Csp3 |
| 1720 | ν C=O carboxylic acids |
| 1658 | δ N-H vibration |
| 1585 | ν C---C Phenyl ring |
| 1511 | ν C---C Phenyl ring |
| 1462 | ν C---C Phenyl ring |
| 1423 | O-H carboxylic acids |
| 1356 | C-O carboxylic acids |
| 1279 | ν C-N |
| 1210 | ν C-O carboxylic acids, and δ N-H |
| 1130 | ν C-O lactone, ν C-N |
| 1073 | ν C-O alcohol, ν C-N |
| 1024 | ν C-O alcohol, ν C-N |
| 874 | γ O-H carboxylic acids |
| 817 | γ O-H carboxylic acids |
| 789 | γ N-H |
| 683 | γ N-H and γ OH alcohols |
Note: stretch vibration (ν), bend vibration (δ in the plane, γ out of plane).
Table 6 lists some bands that can be assigned to molecular fractions as aromatic ring (1585, 1511 and 1462cm-1), phenol or alcohol (3311cm-1), carboxylic group (1720cm-1). Is interesting the observation of δ N-H and γ N-H vibrations, but ν N-H is not visible, may be because of stretch ν O-H. For this reason, it is possible to think that there are some phenolic fractions in the crude extract or alkaloid structure includes this molecular fragment, combined with other parts. Also, is probably that there is a mixture of alkaloids in the crude.
The similarities of IR spectra of bulbs with roots make easier the analysis (figures 4 and 5). Almost all the bands are the same even in powder and extracts (tables 7 and 8).
Table 7 Characteristic IR-bands of Boophone disticha root powder.18
| Frequency (cm-1) | Assignation |
|---|---|
| 3331 | ν O-H carboxylic acids or alcohols |
| 2910 | ν C-H Csp3 |
| 1731 | ν C=O carboxylic acids |
| 1625 | ν C---C Phenyl ring |
| 1240 | C-O carboxylic acids, ν C-N |
| 1034 | ν C-O alcohol, ν C-N |
| 1027 | ν C-O alcohol, ν C-N |
Note: stretch vibration (ν), bend vibration (δ in the plane, γ out of plane).
Table 8 Characteristic IR-bands B. disticha extracted alkaloids from roots.18
| Frequency (cm-1) | Assignation |
|---|---|
| 3323 | ν O-H carboxylic acids or alcohols |
| 2923 | ν C-H Csp3 |
| 1723 | ν C=O carboxylic acids |
| 1654 | δ N-H vibration |
| 1510 | ν C---C Phenyl ring |
| 1425 | ν C---C Phenyl ring |
| 1282 | ν C-N |
| 1214 | ν C-O carboxylic acids, and δ N-H |
| 1074 | ν C-O alcohol, ν C-N |
| 1026 | ν C-O alcohol, ν C-N |
| 874 | γ O-H carboxylic acids |
| 817 | γ O-H carboxylic acids |
| 789 | γ N-H |
| 683 | γ N-H and γ OH alcohols |
Note: stretch vibration (ν), bend vibration (δ in the plane, γ out of plane).
The table 9 shows an abstract of some characteristic bands which are found in all the spectra. The roots and bulb crude extract powders had similar stretches. Bulb alkaloids had the O-H and C-O stretches just as the roots alkaloids kept these stretches. The spectra have characteristic O-H bonds and C-O which are usually of alcohols, phenols and esters but there are also peaks for aromatic amines and primary amines. The literature describes stretch vibration from 1400-1000 for C-N bond, which is usual in alkaloids.18 There are some bands present in the IR spectra being part of the reported alkaloids:10 buphanamine and distchamine with very similar strcuture, crinamidine, crinine. The functional groups are all common to identified structures in literature, which has been elucidated before.6,8
Table 9 Characteristic IR-bands.
| Frequency (cm-1) | Functional groups or possible compounds |
|---|---|
| 3500-3200 | alcohols, phenols, carboxylic acids |
| 1731-1721 | carboxylic acids |
| 1650-1580 | aromatic compounds |
| 1335-1250 | alkaloids |
| 1320-1000 | carboxylic acids, esters, alkaloids |
The difference between the powder and crude alkaloids was in the number of peaks which increased in the alkaloids while there were no significant differences between the bulb and root spectra for both powder and alkaloids.19 IR is not a conclusive technique, but give important clues for further structural determinations.
Conclusions
The study has shown that inner fresh inner layers and roots of B. disticha have significantly similar secondary metabolite profile which may be due to similar compound structures or just same metabolite classes. The proposed use of roots instead of bulbs in traditional medicine so as to conserve the bulb has also been partly shown in this study to be a reasonable conservation activity of such a high-value medicinal plant. The use of the fractionation method was able to show the existence of some alkaloids in non-polar hexane thus also showing the need of quantitative analysis and structure determination of this/these alkaloid(s) if need be. The absence of alkaloids in toluene which had some other phytocompounds shows that it can be used during the purification of alkaloid crude extracts if the solvent used is miscible with toluene. The methods used before to extract and characterize alkaloids can be used determine the alkaloids in roots with confidence as they have been shown to be similar to those in the bulb to some extent. The existence of alkaloids in almost every extract suggests that the use of roots in the treatment of paralysis through topical use may also be due to alkaloids but does call for bioassay guided tests to further understand these metabolites