My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Tecnología Química
On-line version ISSN 2224-6185
RTQ vol.38 no.3 Santiago de Cuba Sept.-Dec. 2018
ARTICULO ORIGINAL
Bioadsorption of Cu 2+ , Ni 2+ , Zn 2+ and Pb 2+ using an activated sludge process
Bioadsorción de Cu 2+ , Ni 2+ , Zn 2+ y Pb 2+ empleando un proceso de lodo activado
MsC. Jaime Dueñas-Moreno 1 , Ing. Jessica Pérez-Regalado Fuentes 1 , Dr. Carlos Menéndez-Gutiérrez 1 , Dra. Ileana Pereda-Reyes 1
1 Centro de Estudios de Ingeniería de Procesos (CIPRO), Facultad de Ingeniería Química, Universidad Tecnológica de La Habana José Antonio Echeverría (Cujae). Avenida 114 No. 11901 entre Ciclo vía y Rotonda. Marianao. La Habana. Cuba. jaimedm89@gmail.com
Abstract
Heavy metals contents can be reduced from wastewater using microorganisms through different mechanisms included under the general denomination of bioadsorption. Bioadsorption of heavy metals consists in a passive, fast and reversible catchment of the metal ions present in an aqueous solution by physic and chemical interactions on aviable or non-a viable biological materials. In the present paper, a mixed culture of microorganisms from an activated sludge process was used to reduce four different heavy metals contents from a synthetic solution. Copper, nickel, zinc and lead were prepared at a range of 5 – 30 mg/L. The bioadsorption intensity was determined at two values of mean cell retention time (sludge age). The results demonstrated that Freundlich's isotherm adjusted well (R 2 > 0.95) , with a relatively strong bioadsorption due to intensity parameter n . Copper, nickel, zinc and lead final contents were 74.60-77.68%; 75.40 -40.26%; 74.54-78.38% and 25.0-31.6% for 5 and 10 days of sludge age respectively with non-significant differences.
Keywords: heavy metals, bioadsorption, Freundlich's isotherm, activated sludge
Resumen
El contenido de metales pesados puede ser reducido de las aguas residuales empleando microorganismos a través de diferentes mecanismos, incluyendo la bioadsorción. La bioadsorción de metales pesados consiste en una captación pasiva, rápida y reversible de iones metálicos presentes en una solución acuosa mediante interacciones físicas y químicas sobre materiales biológicos viables o no viables. En el presente trabajo se utilizó un cultivo mixto de microorganismos procedentes de procesos de lodos activados para remover metales pesados de un residual sintético. Las soluciones de cobre, de níquel, de zinc y plomo se prepararon en el intervalo de concentración entre 5 - 30 mg/L. La intensidad de adsorción se determinó para dos tiempos de retención medio celular (edad del lodo). Los resultados demostraron que la isoterma de Freundlich ajustó bien (R 2 > 0.95) , con una relativa fuerte bioadsorción de acuerdo con la intensidad del parámetro n . La eliminación de cobre, níquel, zinc y plomo fue 74.60-77.68%; 75.40 -40.26%; 74.54-78.38% y 25.0-31.6% , para las edades de lodo de 5 y 10 días respectivamente, sin existir diferencias significativas.
Palabras claves: metales pesados, bioadsorción, Isoterma de Freundlich, lodo activado
INTRODUCTION
The increase concentration of heavy metals in wastewater is mainly due to industrial origin. Growing efforts have been done to diminish such metal concentrations using adequate technologies [1, 2].
Are well known diverse processes to remove heavy metal from wastewater. The adsorption at different media like activated carbon, zeolite, and others compounds of inorganic origin are one of the most studied [3, 4, 5].
Conventional methods to treat effluents containing Cu 2+ , Ni 2+ , Zn 2+ and Pb 2+ ions include traditional physical and physical-chemical treatment [6, 7, 8, 9]. They are costly as a consequence of its high reactive and energy requirements, toxic sludge generation and the secondary contamination that can be produced [10, 11, 12]. The use of methods for heavy metals removal has incomplete results and is inefficient at relatively low metal concentrations (1-100 mg/L) [13, 14].
There are reports of the use of microorganisms or bioadsorbent ( algae, fungi and bacteria) for removing heavy metals from wastewater [8, 15, 16, 17].
Generally, the bioadsorption process can reduce the capital costs by 20%, the operational costs by 36%, and the total treatment costs by 28% compared with conventional systems [18].
The bioadsorption mechanism is complex and still not well understood. It depends on whether the organism is aviable or non-aviable, as well as the type and species of microorganisms. [15] demonstrated that metal ions in a solution are adsorbed on the biomass through Van der Waals interactions. The adsorption isotherms are normally used to study the adsorption processes. Freundlich´s empirical isotherm is one of the most common model that can be applied [19].
The present work is a study of the removal of four of the most common heavy metals present in wastewater using a mixed culture of microorganisms from an activated sludge process.
MATERIALS AND METHODS
Start up and reactor operation with the mixed culture of microorganisms from activated sludge
To obtain a proper amount of biomass, a batch, cylindrical, completely stirred aerobic reactor with 8 L of effective volume, was used. The Hydraulic Retention Time (HRT) was 1 day with 5 and 10 days of sludge age. The biomass used as seed was collected from an activated sludge wastewater treatment plant located at Jibacoa beach near the Havana. The inoculum was adapted to the new conditions during three weeks until constant Volatile Suspended Solids (VSS) and Chemical Oxygen Demand (COD) concentrations were achieved. The reactor was fed daily with synthetic wastewater concentration using a similar composition as referred by [20], as is shown in table 1.
Table 1. Composition of the synthetic wastewater
| Compound | peptone | meat extract | urea | K 2 HPO 4 | NH 4 Cl |
| Concentration (mg/L) | 640 | 440 | 120 | 34 | 6.8 |
The synthetic wastewater had a COD value of 1 200 ± 14 mg/L. The pH was 7.0 ± 0.5. Addition of 0.5 M NaOH and 0.2 M H 2 SO 4 was necessary. The reactor was maintained at room temperature (30 ± 2ºC).
Analytical tests were performed based on APHA, AWWA, WPCF Standard Methods for Water and Wastewater Analyses [21].
Bioadsorption tests
Stock solutions containing Cu 2+ , Ni 2+ , Zn 2+ , and Pb 2+ ions were prepared dissolving copper, nickel, zinc and lead using CuSO 4 ·5H 2 O, NiSO 4 ·6H 2 O, ZnSO 4 ·7H 2 O and Pb(NO 3 ) 2 respectively in distilled water.
Kinetics study
Tests were conducted to determine the contact time to reach equilibrium conditions, adding the respective metal salt solution to 25 mL of activated sludge (mixed liquor). Metal concentration in the mixed liquor was 5 mg/L and pH 7.0 ± 0.5. It was agitated in an IKA-WERKE shaker at 150 rpm, 30 ± 2 °C, at time intervals of: 5, 10, 15, 20, 30, 40, 60 minutes at room temperature. Each heavy metal and sludge age was tested with 21 samples. Whatman No. 42 filters were used to separate the suspended biomass.
Information on the interaction between bioadsorbent and heavy metals can be obtained from kinetics models. In that sense the pseudo-first order kinetics model or Lagergren's model [22] and the pseudo–second order model, can be tested [23, 24, 25].
The pseudo-first order model can be represented through equation 1.

Where:
q e and q t : (mg metal ion/g bioadsorbent): are the metal adsorbed at time t and equilibrium, respectively, k 1 (min -1 ): is the pseudo-first order reaction rate equilibrium constant.
The pseudo–second order model is shown in equation 2:

Where:
k 2 ( g/(mg min ): is the pseudo-second order reaction rate equilibrium constant
The model parameters were calculated by non-linear regression using Statgraphics Centurion V15 program.
To determine the best model adjustment to the kinetic data, two statistics methods for error analysis were used: correlation coefficient (R 2 ) and residual root mean square error (RMSE). Higher values of R 2 and smaller values of RMSE indicate a better fit of the model [24].
Isotherm experiments
Biomass specific bioadsorption capacity was estimated adding metal salt ion solutions in a range interval concentration from 5 to 30 mg/L to 25 mL of sludge from the activated sludge process.
The specific metal ion adsorbed q (mg metal ion/g bioadsorbent) in each case was calculated according to equation 3.

Where:
q (mg metal ion/g bioadsorbent): amount of heavy metal ion adsorbed, C o and C e (mg/L): are the initial and the equilibrium metal ion concentrations respectively; V (L) is the suspension volume and m (g) is the mass of biomass used as bioadsorbent, measured as volatile suspended solids (VSS).
All experiments were carried out by triplicates.
Removal efficiency
Removal efficiency (% Removal) was calculated according to equation 4.
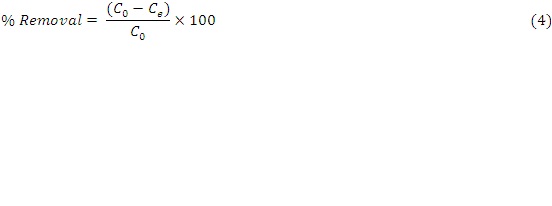
Where:
C o and C e (mg/L): are the initial and the equilibrium metal ion concentrations respectively
Freundlich's Isotherm
In order to determine the bioadsorption isotherm, the experiments were carried out as explained before with the same metal ions concentrations range.
The data obtained was adjusted to Freundlich's isotherm model [19] for both sludge ages, according to equation 5,

Where:
q ads : Specific bioadsorption capacity (mg metal ion/g bioadsorbent); C e (mg/L): equilibrium concentration of metal ions in the solution; k f (L/mg): Freundlich constant related to the bioadsorption capacity and n: Freundlich constant related to the bioadsorption intensity.
The Isotherm's Freundlich parameters were calculated by non-linear regression.
RESULTS AND DISCUSSION
Seed characterization
The seed characteristics are shown in table 2. As it can be seen, the VSS represents the 84% of the Total Suspended Solid (TSS), denoting the excellent sludge quality used as inoculum [27].
Table 2. Seed characterization
| Parameters | Composition (mg/L) |
| Total Solids (TS) | 4 304 ± 48 |
| Total Volatile Solids (TVS) | 3 536 ± 16 |
| Total Fixed Solids (TFS) | 808 ± 31 |
| Total Suspended Solids (TSS) | 4 053 ± 50 |
| Dissolved Fixed Solids (DFS) | 639 ± 33 |
| Volatile Suspended Solids (VSS) | 3 410 ± 17 |
The reactors were considered in steady state conditions when the effluent concentrations in terms of VSS and COD were constant and all tests were carried out. The values of these parameters at steady-state conditions were VSS (mg/L):
1 100 ± 142 and 1 700 ± 128; COD (mg/L): 160 ± 19 and 250 ± 20 for both sludge ages respectively.
Kinetic study
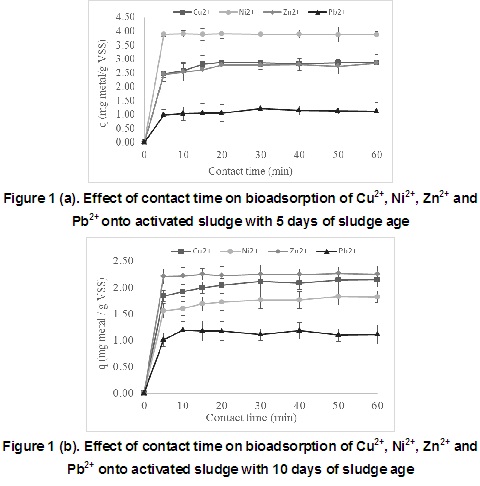
Kinetic study is a very important test in a bioadsorption process, because it describes the rate of bioadsorption. Therefore, the effects of contact time of copper, nickel, zinc and lead solutions onto activated sludge is shown in Figure 1 (a and b) at two values of sludge age (5 and 10 days) at the initial metal concentration of 5 mg/L and 30 ± 2 °C at 150 rpm with a contact time from 5 to 60 minutes.
According to Figure 1, the bioadsorption capacity increases with the increase of the contact time. Initially, all active sites on the bioadsorbent surface are supposed not occupied and the metal solution concentration is high. After that, the surface active sites are increasingly occupied with the time until attaining an equilibrium between the solid and liquid phase. According to this, a contact time of 15 minutes was selected.
In comparison with other kinetic studies using biological adsorbent reported in table 3, this contact time is lower and may be indicating that the bioadsorption mechanism was superficial [28].
Table 3.Summary of equilibrium time from diverse literature
| Bioadsorbent | Metal ions | Metal concentration (mg/L) | Bioadsorbent concentration or mass | Equilibrium time (min) | References |
| Marula seed husk (Sclerocary abirrea) | Pb 2+ and Cu 2+ | 5 | 800 mg | 20 and 180 | [12] |
| Sophora japónica podspowder | Ni 2+ , Zn 2+ and Pb 2+ | 5 | 5000 mg/L | 120 | [29] |
| Saccharomyces Cerevisiae | Cu 2+ , Zn 2+ and Pb 2+ | 10 | 1000 mg/L | 30 | [28] |
| Otostegia persica | Pb 2+ | 5 | 1060 mg/L | 40 | [30] |
| Natural lignocellulosic agro-industrial | Zn 2+ | 3000 | 5000 mg | 180 | [26] |
| Extracellular polymeric substances extracted From aerobic granular sludge | Pb 2+ and Zn 2+ | 10 and 100 | 2000 mg | 360 | [31] |
| Tree sawdust. eggshell and sugarcane bagasse (SB) | Cu 2+ , Pb 2+ and Zn 2+ | 10 | 100 mg/L | 90 | [32] |
| Activated Sludge (5 and 10 days the sludge ages) | Cu 2+ , Ni 2+, Zn 2+ and Pb 2+ | 5 | 1100 and 1700 mg/L | 15 | This study |
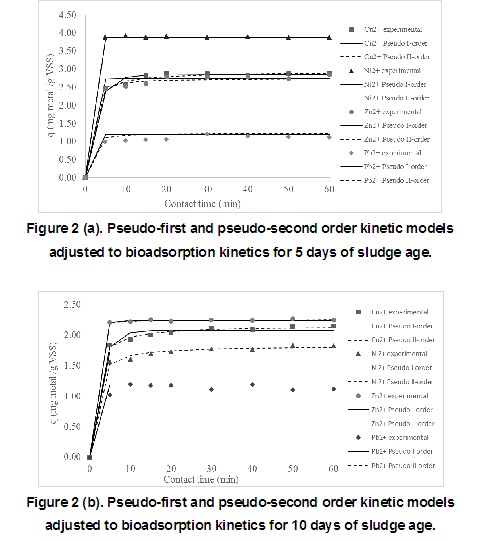
Bioadsorption kinetics of cooper, nickel, zinc and lead solutions ions were analysed with two different adsorption models (pseudo-first and pseudo-second order). Pseudo first and second order models were adjusted to the results as graphically are shown in Figure 2 (a, b). The model parameters were calculated using non-linear regression.
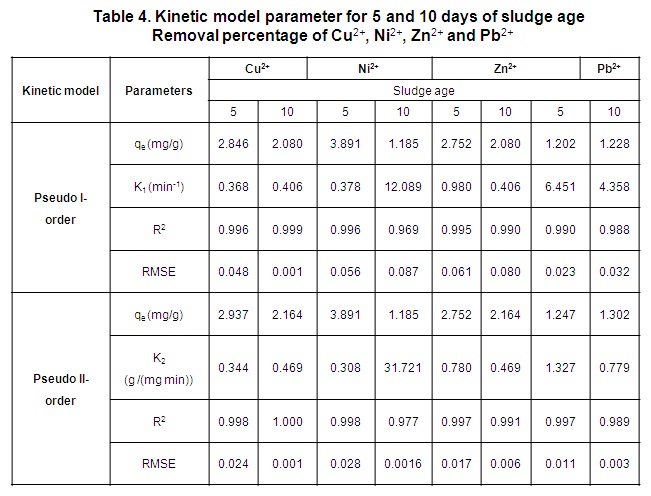
In table 4, the correlation parameter values for both models (pseudo-first order and pseudo–second order) is shown for each method used to evaluate the metals. According to the results, a good adjustment was obtained for all cases with similar values of R 2 and RMSE. As a consequence, both models can be used to predict the kinetics of the bioadsorption process. When considering the short time obtained to reach the equilibrium, it can be assumed that physical bioadsorption occurred. This result is similar to the one obtained [22, 33, 34].
The percentage of metal ions removed once the equilibrium condition was reached, is shown in table 5, for 5 and 10 days of sludge age. The higher removal percentage were obtained for Cu 2+ , Ni 2+ , Zn 2+ at 5 days of activated sludge ; and Cu 2+ and Zn 2+ at 10 days of activated sludge. In all experiments, Pb 2+ was more resistant to bioadsorption.
Table 5. Metal ions removal at equilibrium condition
|
| Sludge age: 5 d and VSS: 1100 ± 142 mg/L | Sludge age: 10 d and VSS: 1700 ± 120 mg/L |
| Metal | % Removal | % Removal |
| Cu 2+ | 74.60 | 77.68 |
| Ni 2+ | 75.4 | 40.26 |
| Zn 2+ | 74.54 | 78.38 |
| Pb 2+ | 25.0 | 31.6 |
Similar result was obtained by [35] and [36]. However, in these papers, the removal percentages of lead solution ion were higher. Considering that bioadsorption is influenced by different parameters like: surface properties and affinity of bioadsorbent for different heavy metal ions [37], different orders can be obtained in different bioadsorption studies. In table 6, is shows the removal percentages obtained by other authors in similar cases. Nearly all removal percentage are lower than removal percentage obtained in this paper. In general, n o significant difference in the removal percentage was found for 5 and 10 days of sludge age.
Table 6. Summary of removal percentage obtained by other authors
| Bioadsorbent | Metal ions | Removal Percentage (%) | References |
| Mesoporous silica on multi-walled carbon nanotubes | Cu 2+ , Ni 2+ , Zn 2+ and Pb 2+ | 45.0, 20.0, 25.0 and 75.0 | [35] |
| Pleurotus ostreatus | Cu 2+ | 56.56 | [36] |
| Liquid laboratory chemical waste by Pleurotus ostreatus | Cu 2+ , Zn 2+ and Pb 2+ | 14.7, 12.24 and 20.4 | [37] |
| Nostoc muscorum | Cu 2+ and Zn 2+ | 96.42 and 74.43 | [39] |
Isotherm of bioadsorption
Figure 3 (a, b) shows the experimental bioadsorption results and the corresponding Freundlich's isotherm for the data in the range of metal ions concentration from 5 to 30 mg/L. The isotherm is a type II shaped. It may indicate a physic multilayer bioadsorption. This suggests that bioadsorption of Cu 2+ , Ni 2+ , Zn 2+ and Pb 2+ was more likely a heterogeneous surfaced adsorption, instead of a monolayer sorption [14] .
The parameters of the Freundlich model, adjusted by a none linear regression, are shown in Table 7. The bioadsorption intensity, n values where between 0 and 10, suggesting relatively strong bioadsorption [15] (Akbari et al., 2015) of these ions onto the surface of the activated sludge for each sludge ages . According to the magnitudes of n value, the bioadsorption intensity orders were: Ni 2+ > Zn 2+ > Cu 2+ > Pb 2+ and Zn 2+ > Cu 2+ > Ni 2+ > Pb 2+ for 5 and 10 sludge age respectively.
An ANOVA analysis of the obtained results shows that the age of sludge had not significant effect with 95% of confidence level (P-value > 0.05) (Table 8).
Table 8. Analysis of variance for the intensity of bioadsorption
| Fountain | Sum of Squares | DF | Mean Square medium | F-ratio | P-value |
| Main Effects |
| ||||
| A: Age | 0.00781 | 1 | 0.007812 | 0.06 | 0.8220 |
| B: Metal | 0.66753 | 3 | 0.222513 | 1.71 | 0.3343 |
| Waste | 0.38923 | 3 | 0.129746 |
| |
| TOTAL (Corrected) | 1.06459 | 7 |
| ||
All F-ratios are based on the mean square residual error
Conclusions
The sludge obtained from the activated sludge process at 5 and 10 days of sludge age demonstrated be a good bioadsorbent for Cu 2+ , Ni 2+ , Zn 2+ removal at a concentration interval of the studied metal ion solution. The time to attain the adsorption equilibrium conditions was considered 15 minutes in all cases. This short time obtained to reach the equilibrium suggests that physical bioadsorption occurred.
Both models, pseudo-first order and pseudo-second order can be used to predict the kinetics of the bioadsorption process. The higher removal were obtained for Cu 2+ , Ni 2+ , Zn 2+ at 5 days of activated sludge ; and Cu 2+ and Zn 2+ at 10 days of activated sludge. In all experiments, Pb 2+ was more resistant to bioadsorption. The bioadsorption intensity orders were: Ni 2+ > Zn 2+ > Cu 2+ > Pb 2+ and Zn 2+ > Cu 2+ > Ni 2+ > Pb 2+ for 5 and 10 sludge age respectively; the age of sludge had not significant influence onto biosorption intensity.
Bibliography
1. ARECO, María; HANELA, Sergio; DURAN, Jorge; DOS SANTOS, María. Biosorption of Cu (II), Zn (II), Cd (II) and Pb (II) by dead biomasses of green alga Ulva lactuca and the development of a sustainable matrix for adsorption implementation. Journal of Hazardous Materials. 2012, vol 213, p.123-132.
2. FU, Rongbing; YANG, Yingpin; XU, Zhen; ZHANG, Xian; GUO, Xiaopin; BI, Dongsu. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere . 2015, vol 138, p. 726-734.
3. BLANCO, F. Alien; ORTEGA, H. Luis Ibraín; DUEÑAS, M. Jaime; BATISTA, G. Rolando; SERAFÍN, P. Ricardo; AUTIÉ, P. Miguel Armando. Remoció n de plomo (II) en vidrio volcánico y propuesta de adsorbedor por etapas. Revista Internacional de Contaminación Ambiental . 2014, vol 30 N° 2, p. 167-175.
5. FEDOROCKOVÁ, Alena; SUCIK, Gabriel; RASCHMAN, Pavel. Activated Zeolite and Magnesite as Potential Reactive Materials for Passive Acidic Groundwater Treatment Technology. Solid State Phenomena. 2016, vol 244, p. 221-227.
8. TONETTI, Cinzia; ALUIGI, Annalisa; SELMIN, Francesca; CILURZO, Francesco; MAZZUCHETTI, Giorgio. Removal of Cu (II) ions from water using thermally-treated horn–hoof powder as biosorbent. Desalination and Water Treatment. 2014, vol 55, N° 4, p. 1-11.
13. BAJPAI, AK; RAI; Leena. Removal of chromium ions from aqueous solution by biosorption on to ternary biopolymeric microspheres. Indian Journal of Chemical Technology . 2010, vol 17, p. 17-27.
14. CAO, Yan-ru; LIU, Zhuan; CHENG, Guang-lei; JING Xiao-bing; XU Heng. Exploring single and multi-metal biosorption by immobilized spent Tricholoma lobayense using multi-step response surface methodology. Chemical Engineering Journal. 2010, vol 164, N° 1, p. 183-195.
19. FREUNDLICH, H. Over the adsorption in solution. J. Phys. Chem . 1906, vol 57, p. 385–407.
20. OLIVEIRA, Catarina; ORDAZ, Alberto; FERREIRA, Eugénio; ALVES, Madalena; THALASSO, Frédéric. In situ pulse respirometric methods for the stimation of kinetic and stoichiometric parameters in aerobic microbial communities. Biochemical Engineering Journal . 2011, vol 58-59, p. 12-19.
25. HO, Y.S; MCKAy G. Pseudo-second-order model for sorption processes. Process Biochemestry. 1999, vol 34, N° 5, p. 451–465.
26. ABDOLALI, A; NGO, H.H; GUO, W.S; LEE, D.J; TUNG, K.L; WANG X.C. Development and evaluation of a new multi-metal binding biosorbent. Bioresource Technology. 2014, vol 160, p. 98-160.
27. AHN, Dae-Hee; CHANG, Won-Seok; YOON, Tai-lI. Dyestuff wastewater treatment using chemical oxidation, physical adsorption and fixed bed biofilm process. Process biochemistry. 1999, vol 34, N° 5, p. 429-439.
30. ALAVI, S.A; ZILOUEI, H; ASADINEZHAD, A. Otostegia persica biomass as a new biosorbent for the removal of lead from aqueous solutions. I International Journal of Environmental Science and Technology. 2015, vol 12, N° 2, p. 489–498.
32. PUTRA, Wiwid Pranata; KAMARI, Azlan; YUSOFF, Siti Najiah Mohd; ISHAK, Che Fauziah; MOHAMED, Azmi; HASHIM, Norhayati; ISA, Illyas Md Biosorption of Cu (II), Pb (II) and Zn (II) ions from aqueous solutions using selected waste materials: Adsorption and characterisation studies. Journal of Encapsulation and Adsorption Sciences. 2014 , vol 4, p. 25-35
35. YANG, Weijie; DING, Ping; ZHOU, Lei; YU, Jingang; CHEN, Xiaoqing; JIAO, Feipeng. Preparation of diamine modified mesoporous silica on multi-walled carbon nanotubes for the adsorption of heavy metals in aqueous solution. Applied Surface Science. 2013, vol 282, p. 38-45.
36. RAFIQ, Sohail; ALI, Majid, K.M; SAKARI, Mahyar; SULAIMAN, Jumat YASIR, Suhaimi. M. Biosorption of Toxic Heavy Metals by Unmodified Marine Red Alga (Kappaphycus alvarezii): Kinetics and Isotherm Studies. International Journal of Environment and Bioenergy. 2013, vol 7, N° 2, p. 91-107.
37. BULGARIU, Dumitru; BULGARIU, Laura. Equilibrium and kinetics studies of heavy metal ions biosorption on green algae waste biomass. Bioresource Technology . 2012, vol 103, N° 1, p. 489–493.
38. ARBANAH, Muhammad; NAJWA, Miradatul; HALIM, Ku. Biosorption of Cr (III), Fe (II), Cu (II), Zn (II) ions from liquid laboratory chemical waste by Pleurotus ostreatus. International Journal of Biotechnology for Wellness Industries. 2012, vol 1, N° 3, p. 152-160.
Recibido: Marzo 2018
Aprobado:Julio 2018
MsC. Jaime Dueñas-Moreno. Centro de Estudios de Ingeniería de Procesos (CIPRO)