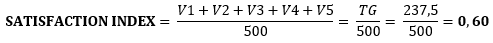Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Cooperativismo y Desarrollo
versión On-line ISSN 2310-340X
Coodes vol.11 no.2 Pinar del Río mayo.-ago. 2023 Epub 30-Ago-2023
Original article
Design and implementation of an integrated management system for livestock enterprises
1 Empresa Pecuaria Genética "Camilo Cienfuegos". Consolación del Sur, Pinar del Río, Cuba.
2 Universidad de Pinar del Río "Hermanos Saíz Montes de Oca". Facultad de Ciencias Económicas. Centro de Estudios de Dirección, Desarrollo Local, Turismo y Cooperativismo. Pinar del Río, Cuba.
The livestock industry plays a fundamental role in our society, providing food of animal origin and contributing to economic and environmental sustainability. However, it faces significant challenges in the area of management, such as the lack of integration of processes, the lack of adequate technological tools and the difficulty in obtaining accurate data in real time. The objective of this article is to present a procedure for the design and implementation of the Integrated Management System for livestock enterprises that will contribute to greater efficiency in decision making by management and to the improvement of its capacity to react to new needs or expectations of stakeholders. For the development of the research, theoretical methods were used with emphasis on the systemic and qualitative approaches, while as empirical methods, techniques such as process mapping, flow charting and checklists, among others were used. Among the main results obtained a procedure outstands, which, by adopting the process-based approach and the Plan-Do-Check-Act cycle, makes it possible to deploy an intervention project in the enterprise under study for the implementation of the Integrated Management System, which scope are the quality and food safety management systems, respectively. The main conclusions are the increase in customer satisfaction levels and the improvement of other performance indicators with efficiency and effectiveness criteria.
Key words: meat production; quality management system; food safety management system; integrated management system
Introduction
International trade in food products in all spheres of society has an increasing trend, providing important social and economic benefits. This also facilitates the spread of diseases in the world. Since food consumption habits have also undergone significant changes in many countries during the last decades and, consequently, new techniques of food production, preparation and distribution have been perfected (González Enríquez & García Pérez, 2022). Therefore, effective hygiene control is essential in order to avoid the detrimental consequences of foodborne illness and food spoilage, both for health and the economy.
On the other hand, the culture of quality implies the adoption of the sustainability approach. In the case of food production, it is not enough to offer a product that meets the requirements of production standards if it harms the people involved in production, the detriment of environmental conditions or the failure to take into account the criteria of all stakeholders, including customers (Castell Catalá & de la Nuez Hernández, 2021).
In that sense, enterprises need to implement an Integrated Management System (IMS) that encompasses the quality and food safety subsystems, which will help the organization to meet the requirements of its customers in terms of the product and ensure food safety, in a logical and objective way to generate customer satisfaction (Quiceno Giraldo & Ángel Álvarez, 2014).
Since its inception, the management of the systems by the enterprises has been done independently, with the passage of time it has been shown duplication in documentation and higher cost and time for management. For these reasons, it is recognized the need to integrate these systems to be more efficient and effective, being at the same time necessary to unify efforts among all stakeholders of the organization (Sanabria Torres et al., 2020).
A Quality Management System is that "part of a management system related to quality" (ISO, 2015), it implies a set of standards that interrelate with each other to enforce the quality requirements that an enterprise demands to meet the requirements agreed with its customers through continuous improvement, in an orderly and systematic manner (Vásquez Lema & Vázquez Loaiza, 2021).
For its part, a Food Safety Management System is a systematic approach to controlling food safety hazards to ensure that food is safe for consumption. All enterprises are required to establish, implement and maintain this system based on the principles of Hazard Analysis and Critical Control Points (HACCP). In the development of this research, the concepts of food safety and quality that are taken into account focus on the postulates of the quality gurus: Philip Crosby, William Edwards Deming, Kaoru Ishikawa, Joseph Juran and Armand Vallin Feigenbaum, influential authors on the subject and its management (Leal Rodríguez et al., 2021).
HACCP is a food safety system based on the identification of all potential hazards in ingredients and different food production processes (González Díaz et al., 2023). As the introduction of food safety hazards can occur at any point in the food chain, proper control of each link in the food chain is essential. Thus, food safety is ensured through the combined efforts of all parties involved in the food chain (ISO, 2018).
In that sense, ISO 22000 is an international standard on Food Safety Management System for the supply chain, ranging from farmers and ranchers, processors and packaging, to transportation and point of sale. This standard focuses on securing the supply chain, presents principles for integrated management systems and is aligned with the HACCP principles of the Codex Alimentarius1, it is designed to be implemented in any type of organization, regardless of the type of enterprise, size, sector and geographical location it has (ISO, 2018).
The way to ensure that food does not cause harm to consumers is to have food safety management systems incorporated into the food chain processes. Every actor in the food chain must have "a food hygiene system", which, as established by the Codex Alimentarius, includes prerequisite programs complemented by control measures at Critical Control Points, as appropriate, which together ensure that food is safe and suitable for its intended use (PAHO, 2020).
For their part, the ISO 9000 series standards are the most widely used standards for quality assurance in the food sector, so the current trend is to combine the potential of ISO 9000 with the HACCP system, since both are based on a political decision of the organization, involve all the enterprise's personnel, have a clearly structured approach and require clearly specifying the key aspects in the processes to achieve each one's purpose.
Although no standards have yet been developed that establish the requirements for an integrated management system, it is worth noting that with integration, better planning, management and control of all the organization's activities is achieved. In addition, a more competitive position in the market is achieved, by offering products with requirements included in several international standards, thus ensuring greater reliability on the part of customers.
In 2008, in Cuba, the NC PAS 99:2008 standard was introduced, a guide that provides consultants advising organizations with guidelines for the implementation of an integrated management system covering quality, environment and occupational health and safety. This guide is based on the specific requirements of the management system standards and on the common requirements for these systems as a framework for integration.
The PAS 99:2008 NC, like any other standard, sets out what to do without providing tools or techniques to evaluate all the above aspects. This should be the task of the organizations that decide to implement it, which means that the diagnostic phase does not appear explicitly.
Similarly, there is a greater trend towards the integration of the environmental management system based on the NC ISO 14001:2015 and the Quality Management System, according to the NC ISO 9001:2015, although this has been extended towards the integration of the Occupational Health and Safety System with reference to the NC 45001:2018 and the Internal Control System based on Resolution 60/2011 of the Office of the Comptroller General of the Republic (Gómez et al., 2018).
In the case of food producing companies, it is mandatory to ensure the health of customers who consume their products. To this end, NC 136:2017 has been established, which sets out the requirements for a Hazard Analysis and Critical Control Point System. The HACCP system constitutes a scientific and systematic approach, applied in the food industry for the identification of specific hazards related to consumption and food safety. As part of the system, programs are designed that include incoming raw materials and materials, in-process and finished production (Sung-Won et al., 2019).
Therefore, it is agreed with several sources (Council of State, 2020; ONN, 2017; Sung-Won et al., 2019) that it is important to implement this system along the entire food chain, since it is conducive to control and decrease the inherent risks of certain foods or processing procedures, as the microbiological sensitivity and potential physical and chemical contaminants of some products mean that any deviation has negative effects on the health of consumers.
The Genetic Livestock Enterprise "Camilo Cienfuegos", despite initiating the first steps in the design of documented information related to its quality management system in the first place, and to its food safety management system, has not achieved the certification of any of them separately, based on the latest versions of the Cuban standards (NC) ISO 9001:2015 and NC 136:2017, so it is facing the current challenge of finding possible alternatives to guarantee the safety and innocuousness of its productions while increasing, at the same time, productivity and quality through integrated management.
The fact that the management of business systems has so far been conceived independently has not allowed for better control of the processes and, consequently, has affected the increase of its competitiveness, since the image projected both externally and internally has deteriorated.
The general objective is defined as: to present a procedure for the design and implementation of the Integrated Management System in Genetic Livestock Enterprise "Camilo Cienfuegos" that contributes to greater efficiency in decision making by the management and to the improvement of its capacity to react to the new needs or expectations of the interested parties.
Materials and methods
The main information needs in the present research are related, in the first place, to the regularities identified regarding the predominance of the different management approaches to carry out the management of business systems, that is, what is the approach that has predominated so far in terms of systems management (integrated or not), what is the degree of implementation of management systems, what are the regulations available to undertake a process of integration of management systems in the enterprise, among others.
The objective of the diagnosis was to identify the limitations that are present in the management process of the business systems, particularly those that take place in the scope of the processes that are developed in the "El Canal" packing plant, the observation unit defined by the authors to contribute to the solution of the research problem formulated.
Secondary sources of information include documents describing the enterprise's global strategy towards 2030, balance sheet reports for the last 3 years, the enterprise's improvement dossier, quality manual, procedures, work instructions, safety manual, enterprise standards, internal records and other documented information related to the production of fresh produce and sausages in general. As for the primary sources of information, they include subjects as clients, managers and workers of the enterprise, as well as specialists from the Higher Organization of Business Management, the National Bureau of Standardization and other interested parties.
The design of formats for the collection of information includes a questionnaire in the form of a survey to be applied to the enterprise's current customers, a self-administered questionnaire (survey) to workers and an interview with managers. In addition, three checklists are used that were developed by the authors, based on the content of the NC ISO 9001:2015 and NC 136:2017 standards.
The sample.exe program was used to determine the sample size for the employee questionnaire, which offers a reliability of 95% and a minimum permissible margin of error of 0.10. In the case of the client survey, it was applied to 100 % of clients (9 entities). Similarly, an interview was conducted with 100 % of the managers.
In data processing, automated procedures are combined with the help of Statistical Package for Social Sciences and MiniTab software (both computer programs designed to perform basic and advanced statistical functions) and the expertise of specialists in research techniques.
Results and discussion
From the documentary analysis, it is possible to refer the following general information about the organization under study: the Genetic Livestock Enterprise "Camilo Cienfuegos" integrated to the Livestock Business Group, subordinated to the Ministry of Agriculture is located in the "Corralitos" farm, in Entronque de Herradura , municipality of Consolación del Sur in the province of Pinar del Río. The enterprise has a manual of Good Manufacturing Practices, a hygienic-sanitary manual according to NC 143:2010 - Code of Practices - General Principles of Food Hygiene to the processes covered by the food safety system, documents that guarantee the Good Hygiene Practices of the organization, likewise, it also has a manual of complementary documents that support the HACCP system.
The entity also has a quality manual, developed from the requirements of the NC ISO 9001:2015 - Quality Management Systems (QMS). Requirements, which is supported by documented information contained in procedures, work instructions, product standards, quality inspection standards, methodologies, records and other documentation related to the QMS.
In addition, the enterprise has a manual of standard operating procedures related to quality and a safety manual based on the Hazard Analysis and Critical Control Point system.
At the risk of considering the results of the analysis of information from secondary sources as insufficient, several instruments were applied to collect information from primary sources regarding the current situation of the enterprise with respect to the degree of implementation of both the QMS and the HACCP system.
In order to measure the customer satisfaction index for the product offered, a survey was applied (Annex) based on a methodology, as cited in Castell Catalá and De la Nuez Hernández 2021). As a result of this survey applied to a sample of 9 subjects, a value was obtained that reflects a deterioration of the satisfaction index as follows: 0.60; the product attributes that most influence this result are precisely: texture, smell and freedom from undesirable parts.
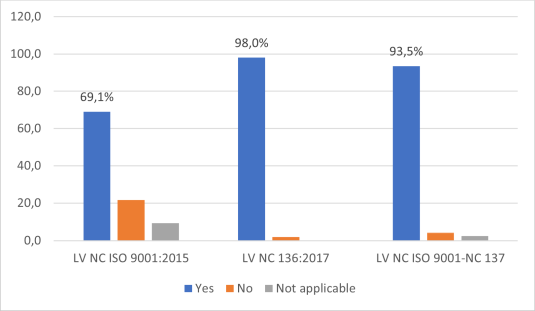
In order to deepen the analysis of the current situation related to the level of implementation of both systems (quality and safety), three checklists were applied, which were developed taking into account the requirements of the reference standards (NC ISO 9001:2015 and NC 136:2017). Figure 1 shows the results of the application of the three checklists, expressed as a percentage of compliance with the requirements of each standard by the enterprise.
 Source: Prepared by the authors
Source: Prepared by the authorsFigure 1 Degree of compliance with the requirements according to the checklists applied
When applying the checklist according to NC ISO 9001:2015, 69.1 % compliance of the evaluated aspects is obtained, while in the verification according to NC 136:2017, 98 % is recorded and with respect to the checklist applied for Hazard Analysis and Critical Control Point (HACCP) and NC ISO 9001:2015 audits, 93.5 % compliance is recorded.
At the conclusion of the diagnosis, common aspects stand out as a result of the application of the research techniques used to characterize the current state of the systems management process (quality and food safety) in the Genetic Livestock Enterprise "Camilo Cienfuegos", such aspects are related to the following:
The organization has a quality policy aligned with the global strategy, but it does not include aspects related to food safety, and therefore does not consider the integration of both systems.
It is also evident that the quality objectives are neither measurable nor consistent with the policy, nor are they conceived in an integrated manner with the food safety system.
The main problems affecting product quality and safety are: technology obsolescence, shortage of spare parts and unsatisfactory conditions of the organization's infrastructure.
The documentation associated with the HACCP system is not integrated with the quality management system.
The prevailing culture to date encourages independent management of business systems.
By way of discussion, it is observed as a generality, that the bibliographic sources consulted (González Enríquez & García Pérez, 2022; PAHO, 2020; Quiceno Giraldo & Ángel Álvarez, 2014; Sanabria Torres et al., 2020) use a diagnostic methodology based on the reference standards. It allows defining, measuring, analyzing and controlling the existing gaps in the organization for the implementation of an integrated management system with scope in the quality and safety management systems respectively and during the production process, thus seeking to increase customer satisfaction by offering quality products in the shortest possible time.
In the research analyzed on quality management and food safety management (González Enríquez & García Pérez, 2022), it is indicated that the design of the HACCP system must include all the safety measures established by the Codex Alimentarius, the purpose of which is to guarantee safe and quality food to all people everywhere. The central focus of each of the measures adopted is to eliminate or minimize, to acceptable levels, the critical control points to ensure product safety and raise the level of consumer confidence. González Enríquez and García Pérez (2022) agree when they state that the implementation of Good Manufacturing Practices is fundamental for the construction of safety management systems such as Hazard Analysis and Critical Control Points.
Likewise, González Enríquez and García Pérez (2022) agree that both systems (quality and safety) are perfectly integrable since they have in common the fact that their structure is based on the Deming PHVA cycle (Plan, Do, Check and Act), where the activities of the design process are planned, the activities planned to design and implement the proposed system are executed, the effectiveness of the implemented system is reviewed and measured and, finally, measures are taken to improve or eliminate the deficiencies detected in the review and follow-up actions, thus complying with the phases of the cycle.
To contribute to the solution of the identified problem associated with the integration of business management systems, a procedure is proposed for the design and implementation of the IMS, based on the continuous improvement cycle or Deming cycle, which facilitates the fulfillment of the formulated objective, by integrating the recommendations emanating from the Cuban Standards NC PAS 99:2008 and the requirements established in the standards NC ISO 9001:2015 - Quality Management Systems and in NC 136:2017 - Hazard Analysis and Critical Control Point System and guidelines for its application.
Procedure for the design and implementation of the Integrated Management System in livestock enterprises
The objective of the proposed procedure is to establish a specified way to achieve integration, based on a sequence of steps that harmoniously relate the requirements of each management system with those of the ISO 9001:2015 NC, adopting the process-based approach as the backbone and organizer of the management activity. This contributes to reduce or eliminate the vision that predominates in terms of their separate management and is focused so that all systems are managed as a whole from the common requirements, without losing their specificities.
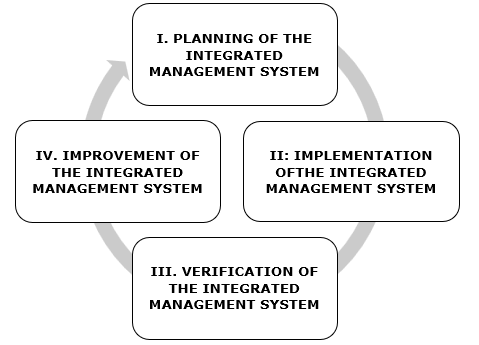
The following is a description of the proposed procedure for designing and implementing the IMS of the management systems implemented (Quality and Safety), based on complying, in a unique manner, with all the requirements declared as common. This is structured in four phases, in correspondence with the Plan-Do-Check-Act cycle, recommended for all management systems.
The IMS design and implementation process in livestock enterprises comprises four phases (Figure 2).
 Source: Prepared by the authors
Source: Prepared by the authorsFigure 2 Sequence of the process to design and implement the Integrated Management System in livestock enterprises
The following is a description of the content of each of the phases for carrying out the process related to the integration of the management systems selected by the enterprise.
Phase I (Planning): Planning of the integrated management system
The objective of this phase is to organize the design and implementation process of the IMS through the development of a work schedule, taking into account the result of the diagnosis, the current degree of integration and the necessary actions to harmoniously relate the elements established in the requirements set forth in the certifiable standards (NC ISO 9001:2015 and NC 136:2017). An integration plan is prepared that includes the activities to be carried out to achieve the outlined objective; it must contain the estimated duration of each one of them, start date, responsible and completion date, the resources to be used and partial evaluation cuts to verify the progress of such activities.
Phase II (Doing): Implementation of the integrated management system
The objective of this phase is the implementation of the documented information that is elaborated. The documentary structure of the IMS is conformed, which includes the Business Management Manual, integrated policy, process sheets, procedures, records, instructions; the mission, vision, strategic objectives, organizational structure, process map and other information related to the IMS required by the application standards or considered by the enterprise are ratified or reformulated; the documentation is unique for all systems and specific procedures and records are available for each standard in question. Risks are also identified, levels of responsibility and authority are established, exclusions from the requirements of the NC ISO 9001:2015, as required in the case of the enterprise, all of which is subject to review and approval by senior management and subsequently communicated to workers for their knowledge and involvement in the performance of system activities. It is at this stage that the expected results materialize, it is where the change must really be achieved, therefore, the process managers play a fundamental role in the management of change in organizational behavior, culture and in the reinforcement of the values of the group.
Phase III (Verify): Verification of the integrated management system
The purpose of this phase is to evaluate the effectiveness of the system by defining the necessary modifications to be made according to the information, documentation related to the IMS, according to the results obtained in the controls carried out. An agenda item related to the progress of the implementation of the IMS in the enterprise is included in every board meeting.
Phase IV (Action): Improvement of the integrated management system
The result of this phase is to guarantee the improvement of the system by implementing corrective actions to achieve the planned results and update the documentation, seeking to eradicate the causes of nonconformities detected during audits or reviews of the system.
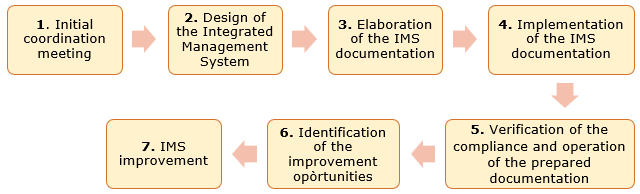
The implementation of the IMS design and implementation process in livestock enterprises is based on the following procedure comprising the following steps (Figure 3):
The content of each step is described below:
Step 1: Initial coordination meeting
Objective: To approve the implementation of the IMS, since it is a project that requires significant human, financial and technical resources.
Responsible parties: senior management (full board of directors) and consultants, if deemed appropriate by the enterprise.
Activities:
Establishment of the organization of the work to be carried out by the members of the IMS project team to be undertaken in the enterprise. The IMS project coordinator (Deputy Director) chairs the work team.
Conducting the diagnosis with a participatory approach and a global vision of the enterprise and its key factors for success.
Development of a schedule of meetings to evaluate the degree of progress and in which periodic reports are prepared for presentation to the Board of Directors and the "IMS Team".
Conduct an in-depth investigation of the management systems that the enterprise already has in place and make a comparison with those to be implemented in an integrated manner in order to analyze the gap between the starting point in which the organization is and the objective it wants to achieve.
Execution of a timely diagnosis to check whether the current system is capable of supporting the implementation of the IMS. In this activity, the strategies to be followed in the process of implementing the Integrated Management System are also defined and that have the least possible impact within the enterprise, while helping to make the integration of management systems more successful. This diagnostic phase should be carried out with the help of experts (consultants) in these tasks, in collaboration with those members of the organization who have a deep knowledge of the organization. This is one of the steps on which the success of the implementation depends and can last at least one month.
Tools to be used: group dynamics, Gantt chart, relationship matrices.
Step 2: Integrated Management System Design
Objective: Define the IMS structure.
Responsible: IMS Team.
Activities:
Definition of the documented information to be elaborated to comply with the requirements of the IMS based on the results of the diagnosis.
The IMS documentation is structured in three levels according to its importance and rank and must include the documents necessary to respond to the requirements.
First level: manuals: at the macro process level, the Integrated Management System Manual will be prepared, and at the process level, the process manuals will be prepared where appropriate.
Second level: procedures for each process and job profiles.
Third level: other documents (records).
The documentation is arranged as follows:
Business management manual.
Process folder: each process will have its own process folder that includes the process sheet, specific process procedures, process position profiles, effectiveness indicators, corresponding system records and other documents that may be deemed pertinent to include.
In relation to the necessary documented information to be maintained by the enterprise will depend on (ISO, 2018):
Size of the organization and type of activities
Process complexity
Maturity of the management system
Risks and opportunities
Competence of people
Legal and regulatory requirements
Customer and other stakeholder requirements
Need for evidence of results achieved
Need to support remote accessibility and recoverability
In any case, the enterprise should always provide evidence of the following types of documented information: Integrated Policy, Integrated Objectives, IMS Manual, Procedures, Automated Workflows, Work Instructions, Forms, Retained Documents (e.g. records).
Measurement of compliance by means of a meeting to approve the proposed document design. The minutes of the meeting must contain the following information: date, time and place of the meeting, attendees, relevant points of the analysis carried out, deviations detected, corrective actions to be taken, signature and date of the person preparing the report, signature of the general manager. The report shall also include all decisions and actions taken to improve the management system.
Tools to be used: meetings, process mapping, flow charts.
Step 3: Development of the integrated management system documentation
Objective: to document information related to the IMS.
Responsible: Project coordinator and IMS team.
Activities:
The manual is prepared based on the NC ISO 9001:2015 for the QMS and covering in its processes the requirements of NC 136:2017. The process folders are updated, each folder is reviewed, updating the aspects of new inclusion, exclusion or modification that are considered and referencing both standards obtaining as a result the description of each process covering its mission, scope, objectives, customers, interrelationships, activities and sequence of activities through flowcharts, the necessary human, financial, material, documentary and computer resources, responsible for the process, determination of monitoring and measurement requirements, determination of performance indicators and methods for measuring effectiveness. The following aspects must be taken into account when updating the files:
The scope of the process should define what is included in the process, which can be technical, territorial and structural/functional. Answering the question: In which cases does the process apply? The purpose of the process should be concrete and linked to the fundamental objective of the process. Answering the question: What is the raison d'être of the process? The person responsible for the process will have full autonomy of action in its responsibility to respond to the strategic objectives outlined.
To determine which documents control the process will be reviewed:
Regulatory requirements including national and international legislation and standards applicable to the process
Regulations applicable to the process and systems associated with the process
Internal resolutions
System and process-specific procedures
Records (evidence of the operation of the process)
Measurement of compliance through management review of the manual and process sheets. Minutes of the meeting are drawn up to record the approval to continue with the project.
Measurement of compliance through document review by the IMS project coordinator (Deputy Director) to ensure compliance.
To this end, the documentation is socialized among each member of the Board of Directors to take into account their considerations and suggestions. All completed documentation is presented to the board of directors for approval, which is evidenced by the agreements reached and by resolution of the general director.
Tools to be used: group dynamics, process mapping, flow charts.
Step 4: Implementation of IMS Documentation
Objective: To develop a plan for the implementation of the IMS by the same team in charge of the diagnosis, with greater involvement of top management. This plan should include details of the program to be followed, as well as the resources to be allocated.
Responsible: IMS project coordinator and those responsible for the defined processes.
Activities:
Elaboration of the implementation program of the IMS documentation, defining the activities to be executed, the people in charge and the material assurances required, as well as the dates of execution of the same with a view to guaranteeing the execution of the processes and all the documentation prepared.
Approval of the implementation plan by top management at the strategic, tactical and operational levels.
Dissemination of the content of the implementation plan by senior management, as well as by middle management, who inform the rest of the members of the group of their responsibilities, activities, tasks and procedures to be followed to carry out the implementation of the IMS.
Plan implementation.
Tools to be used: group dynamics, process mapping, flow charts, checklists.
Step 5: Verification of compliance and operation of the designed documentation
Objective: to validate the effectiveness of the IMS.
Responsible: Project coordinator and IMS team.
Activities:
Monitoring and measurement of processes and products
Conducting inspections, self-monitoring and/or audits
Management reviews of the IMS
Customer feedback
Tools to be used: group dynamics, survey, relationship matrices.
Step 6: Identification of improvement opportunities
Objective: to determine the opportunities for improvement of the IMS based on the nonconformities detected in the previous step and actions taken by external personnel.
Responsible: Project coordinator and IMS team.
Activities:
Tools to be used: continuous improvement cycle or Deming cycle, benchmarking, brainstorming, cause-effect diagram, checklists.
Step 7: IMS improvement
Objective: to validate the IMS design and implementation procedure.
Responsible: Project coordinator and IMS team.
Activities:
The management takes into account as inputs for the continuous improvement process the results of IMS audits, management reviews, performance of processes and products, measurement of customer satisfaction.
Management reviews of the IMS are planned and executed on a quarterly basis to ensure the continued suitability, adequacy and effectiveness of the system, the opportunities for continuous improvement as an indispensable requirement of any process, the need to adapt the system to the changes that arise, as well as the resources required to do so, are put into practice.
The procedure validation system comprises three criteria:
1. Effectiveness measurement (Results): this criterion evaluates the degree of compliance with the objectives achieved in terms of the number of results obtained, in the following general form:
Effectiveness: Quantity served or actual production / quantity that should have been served or produced
Some examples may include:
Production: Actual production / Planned production
Sales: Actual shipments / Committed shipments
Collections: Monetary units collected / Estimated monetary units to be collected
0 and M: Procedures reviewed / Procedures to be reviewed
Purchases: Orders Placed / Requests Received
Staff: Number of people trained / Number of people to be trained
In addition, the following may be considered:
Assessment of the evolution of the main economic and financial indicators: revenues, costs, profits, wage/value-added ratio, profit/sales, etc.
Results of quality audits and system reviews, established according to reference standards whose objectives are:
2. Efficiency measurement (use of resources): refers to the use made of the resources used in the design and implementation of the IMS. The notion of efficiency best achieved is that which is linked to the increase of the value created, of the value added. That by which the quantity and quality of products is maintained and improved, maintaining and/or reducing the amount of inputs required. This implies that, in order to be efficient, the main focus of attention of the enterprise's management at the operational level in any functional unit must concentrate on the elimination of waste, both visible and hidden, whatever its source. In order to define indicators to achieve efficiency in the use of resources, which could mean a way of quantifying waste, the following will be taken into account:
Unit input requirements (U.I.R.)
Establishment of possible sources of waste leading to increase those requirements
Indicate in general, the form these indicators will take and how to consider them
U.I.R. are the quantities of inputs (whether machinery or equipment, materials, space, energy, man-hours, etc.) required given a process and system or unit capacity, to produce one unit of product or service.
The formula of the U.I.R. is as follows:
U.I.R. = (Quantity of input used) / (quantity of products)
Some examples of U.I.R. are as follows:
Of materials:
(Ton. or batches or units of material X) / (Ton. batches or unit of product)
Machinery:
(Machine hours) / (Tons. or liters produced) = (Computer sheets) / Report
Labor:
(Man hours) / (Tons. or units produced) = (Typing hours) / Report
The U.I.R. is an expression of the use of resources that cannot be dispensed with, since it is used to budget and program the amount of resources needed in a given period, or the cost to be incurred, by multiplying these by the amount of products programmed to be produced and by the prices of the respective inputs.
Quantity of input required = U.I.R. x quantity of products
Input cost = U.I.R. x product quantity x input price
Thus, U.I.R. is also the most widely used way to compare between companies the particular efficiency with which inputs are used and to manage the highest levels of investment, innovation in equipment, new technologies or development of current ones.
At the departmental level, it will be convenient to make run charts of the main U.I.R. (those that have the greatest impact on the unit's cost structure) and identify the reference levels such as historical, standard, design or theoretical, in order to make a more rigorous analysis of the opportunities for improvement.
3. Effectiveness measurement (impact on customer satisfaction): this criterion will assess the impact of what is done, of the product planned, both in terms of quantity and quality, the product that will satisfy the customer or have an impact on the market. It will be measured through:
Evaluation of customer satisfaction: both external (taking into account the requirements of the NC ISO 9001 and the proposed methodology, which assumes that all interested parties are taken into account) and internal (through the results achieved in the participation and commitment of personnel in the integrated management of the systems).
Analysis of records: complaints or claims, corrective actions and treatment of non-conformities.
Assessment of behavioral change of managers and workers: effectiveness of interpersonal communication, ability to collaborate, to work as a team, to resolve conflicts, to take advantage of synergy, etc.
Tools to be used: continuous improvement cycle or Deming cycle, control charts, survey (Annex).
Integrating management systems is a process that requires effort and dedication, but it allows the enterprise that implements it to highly improve its competitiveness by increasing performance and corporate image, thanks to the greater control it provides over the organization.
For the integration of their management systems, it is essential to have a broad knowledge of the organization. It is also necessary that the systems already implemented have a solid structure.
According to the approach followed in this research, it can be posited that in the enterprise, independence prevails between the various management systems (Quality Management System based on NC ISO 9001:2015 and Hazard Analysis and Critical Control Point System according to NC 136:2017) which causes difficulties and duplication of effort.
The proposed procedure leads to a decrease in costs when performing certification audits, as the number of internal audits is reduced, improves system maintenance by using a homogeneous approach in the management of various standards, improves decision making by top management as they have a better understanding of the systems, while less effort is required to integrate new systems.
REFERENCES
Castell Catalá, A., & de la Nuez Hernández, D. (2021). Diagnóstico del subsistema calidad en la Unidad Básica de Producción Cooperativa «Julián Alemán». Cooperativismo y Desarrollo, 9(2), 689-711. https://coodes.upr.edu.cu/index.php/coodes/article/view/424 [ Links ]
Consejo de Estado. (2020). Decreto-Ley Inocuidad alimentaria (Decreto-Ley No. 9). Gaceta Oficial de la República de Cuba, Edición Ordinaria No. 76. https://www.gacetaoficial.gob.cu/es/decreto-ley-9-de-2020-de-consejo-de-estado [ Links ]
Gómez, R. C., Estabil Chalupa, G., Villar Morejón, M. J., & Negrín Sosa, E. (2018). Relevancia de los sistemas integrados de gestión en las entidades petroleras cubanas. COFIN Habana, 12(1), 241-255. https://revistas.uh.cu/cofinhab/article/view/975 [ Links ]
González Díaz, Y., Fernández Aliaga, L., Montes de Oca Abella, O., & Leyva Isaac, C. A. (2023). Análisis de peligros y puntos de control críticos en la UEB Central Azucarero Cristino Naranjo. Tecnología Química, 43(1), 42-58. http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S2224-61852023000100042&lng=es&nrm=iso&tlng=es [ Links ]
González Enríquez, L. R., & García Pérez, E. (2022). Implementación de un sistema de gestión de calidad e inocuidad alimentaria en una comercializadora de alimentos. Conciencia Tecnológica, (63). https://www.redalyc.org/articulo.oa?id=94472192002 [ Links ]
ISO. (2015). Sistemas de gestión de la calidad-Fundamentos y vocabulario (ISO 9000). Organización Internacional de Normalización. https://www.iso.org/obp/ui/#iso:std:iso:9000:ed-4:v1:es [ Links ]
ISO. (2018). Sistemas de gestión de la inocuidad de los alimentos-Requisitos para cualquier organización en la cadena alimentaria (ISO 22000). Organización Internacional de Normalización. https://www.iso.org/obp/ui#iso:std:iso:22000:ed-2:v2:es [ Links ]
Leal Rodríguez, L., González González, A., & Reyes Cañedo, M. (2021). Modelo para la mejora de la calidad alineando las tecnologías de la información y el negocio. COFIN Habana, 15(2). https://revistas.uh.cu/cofinhab/article/view/643 [ Links ]
ONN. (2008). Especificación de requisitos comunes del sistema de gestión como marco para la integración (NC PAS 99). Oficina Nacional de Normalización. https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2008/NC%20PAS%2099%20%20a2008%2026p%20okm.pdf [ Links ]
ONN. (2017). Sistema de Análisis de Peligros y de Puntos Críticos de Control (APPCC/HACCP) y Directrices para su aplicación (NC 136). Oficina Nacional de Normalización. https://ftp.isdi.co.cu/Biblioteca/BIBLIOTECA%20UNIVERSITARIA%20DEL%20ISDI/COLECCION%20DIGITAL%20DE%20NORMAS%20CUBANAS/2007/NC%20136%20%20a2007%20%2017p%20alt.pdf [ Links ]
OPS. (2020). «Reglas de Oro» de la OMS para la preparación higiénica de los alimentos. Organización Panamericana de la Salud. https://www.paho.org/es/emergencias-salud/reglas-oro-oms-para-preparacion-higienica-alimentos [ Links ]
Quiceno Giraldo, L. F., & Ángel Álvarez, B. E. (2014). Diagnóstico del estado de implementación de un sistema integrado de gestión en las unidades productivas asociadas a los Cedezo de la ciudad de Medellín. Revista Ingeniería Industrial UPB, 3(3), 31-41. https://repository.upb.edu.co/handle/20.500.11912/6537 [ Links ]
Sanabria Torres, L. M., Casas Henao, A. del P., Roca Martínez, J. J., Varela Alonso, C. T., Guarín Montenegro, G. F., Rodríguez Rojas, Y. L., Gil Cárdenas, L. M., Roa Hayden, O. C., López Cabrera, C. P., Velásquez Ortiz, V. H., Rodríguez González, L. Y., & Peña Guarín, G. (2020). Investigación en Sistemas de Gestión. Avances y retos de la gestión integral. Universidad Santo Tomás. https://repository.usta.edu.co/handle/11634/23177 [ Links ]
Sung-Won, J., Seok-Hyun, C., Seung-Hee, B., Kong, H. S., & Nam, I. (2019). A study on the improvement of HACCP evaluation items in small-scale meat packaging plant. Korea Journal of Organic Agriculture, 27(4), 437-452. https://eurekamag.com/research/070/470/070470207.php [ Links ]
Vásquez Lema, M. R., & Vázquez Loaiza, J. P. (2021). Liderazgo bajo el enfoque de calidad de los estándares ISO 9000. Revista Boliviana de Administración, 3(2), 75-94. https://doi.org/10.33996/reba.v3i2.7 [ Links ]
1Latin expression meaning "Food Code"; it is an international body created by the Food and Agriculture Organization of the United Nations and the World Health Organization for the development of food safety standards, codes of practice, guidelines and recommendations, the purpose of which is to protect the health of consumers and to ensure fair practices in the food trade.
Annexes
Survey to measure the customer satisfaction index
Dear Customer:
Please answer the following survey according to your perception of your degree of satisfaction with the product we offer.
Thank you in advance for your cooperation.
Received: May 19, 2023; Accepted: August 23, 2023











 texto en
texto en