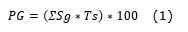Mi SciELO
Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Ciencias Forestales
versión On-line ISSN 2310-3469
Rev cubana ciencias forestales vol.8 no.3 Pinar del Río sept.-dic. 2020 Epub 07-Sep-2020
Original article
Storage methods For Cinchona Pubescens Vahl. Seeds, Imbabura, Ecuador
1Universidad Técnica del Norte. Ecuador.
The management of forest seeds in Ecuador, in relation to their storage, is still insufficient, in particular the Cinchona pubescens Vahl. The objective was to determine the quality of the seeds and the best methods of seed storage of C. pubescens, using different times, containers and conditions. Seed collection was in Intag, Imbabura. The factors under study were: storage time, types of packaging and storage conditions. The quality of the seeds was determined at the time of harvest and in each treatment. Purity reached 76.5 %, the weight of 1,000 seeds was 0.31 g and the humidity content was 14 %. In the first storage trial, no germination was observed in any treatment from the first month. In the second trial, the best storage method was transparent cover stored in refrigeration for one week, which showed 9.25 % germination power. Cinchona pubescens seeds lose their viability quickly, which becomes null after one month of storage.
Keywords: Refrigeration; Containers; Germination; Purity; Humidity content; Germinative vigor.
Introduction
The genus Cinchona, of the botanical family Rubiaceae, known as "cascarilla or cinchona tree" is made up of 23 species (Andersson and Taylor, 1994). It is native to the South American Andes (Garmendía, 2005). It is distributed along the tropical and equatorial zone of the Andes, from 10° North latitude to 20° South latitude. In Ecuador there are 12 of these species, (Ulloa and Jørgensen, 1995), where four are endemic and eight are native. Generally, they are medium to small size trees or shrubs with bitter bark, they can reach a height that can reach 25 meters (Mahecha, et al., 2016). In natural conditions the genus Cinchona presents low rate of germination and regeneration, found only in remote places and in small groups (Buddenhagen et al., 2004).
The species C. pubescens (quinine tree, red quina, cascarilla, quina) is an evergreen tree, 10-25 m high, with a diameter at breast height (DBH) of 20-80 cm in Ecuador (Jugar, 2015). It has the largest geographical distribution area within its genus in the Americas and is found from northern Bolivia to Costa Rica. It is introduced in Tahiti and Hawaii, Asia and in Tanzania, Africa. This species grows at altitudes between 300 and 3300 m (Jäger, 2015), very robust so it was used as a grafting pattern. The total alkaloid content is 3.8 %, of which less than 50 % is quinine. This species is considered rare and endangered in its native range in Ecuador (Günter et al., 2004), while (Jäger and Kowarik, 2010) and (Jäger, 2015), it is considered invasive in the insular conditions of Galapagos.
In the zone of Intag, Imbabura Ecuador, the species C. pubescens is located in relict forests, secondary forests and forming part of silvopastoral systems, at altitudes higher than 1 600 and up to 3 000 m above sea level with a scarce abundance since, according to the inhabitants of the place, its trees are cut down for timber, coinciding with (Gómez, 2016), which poses a similar situation for the District of Kañaris, Lambayeque Region, in Peru.
In the study of forest germplasm supply chains in Ecuador (Prado, Samaniego and Ugarte, 2010), it is stated that there is little availability of quality forest seed in quantity, timely supply to meet producer demand. They also state that there is limited technical and scientific information on the techniques for producing, processing and storing forest seeds of many native species to ensure their viability.
The techniques for the collection, processing and storage of seeds, is a basic condition for designing programs of reforestation, ecological restoration and agroforestry development. Ceballos and López (2007) state that there are few studies on native species, such as the case of the C. pubescens species, on phenological calendars and on the collection and processing (cleaning, drying, humidity content and storage) of forest seeds in Colombia, which is the case in Ecuador. Given the adaptability of this species to the conditions of Intag and other areas of the country, it can be used for various purposes within the country's forestry programs.
Stored seeds are a primary means of production in a country's plant production programs; however, seeds cannot retain their germination capacity indefinitely. The maintenance of their viability depends very much on the storage conditions (Doria, 2010).
In general, the heterogeneity of forest seeds does not allow the same storage technique to be approved, since many show good storage behaviour, while others, on the contrary, deteriorate rapidly under the same conditions. The management of the relative humidity of the air and the temperature of the environment are two key factors to achieve the best results in the storage of the seeds, since they directly influence the speed of breathing of the same ones (Blanco, Durañona and Acosta, 2016).
The cascararilla had a use in the past for its contribution with the quinine alkaloid to act on malaria. The most used species was C. officinalis, while the first species used was C. pubescens. On this basis of knowledge, chemical was synthesized. The virus that causes malaria has mutated and therefore new synthetic products are required, but it is also necessary to know the chemical compounds of other species of Cinchona, so the study of C. pubescens. It is necessary then, to know different aspects of its physiology, among others: time of fructification, viability of the seeds and the conditions of storage that allow to have a successful germination of the same ones.
The objective was to determine the quality of the seeds and the best method(s) to store the seeds of C. pubescens, collected in the area of Intag, Northwest of the Ecuadorian Andes.
Materials and methods
The collection of fruits was carried out in the town of Pucará Alto, Intag, Imbabura, Ecuador, where 30 individuals were selected at random as candidate trees in a silvopastoral system, which were marked and georeferenced. The phenology of the species was carried out through direct observation in the field and the most adequate moment for the collection of the fruits was determined, which was carried out in the month of September, taking into account the relationship of the color of these with their state of ripeness, which corresponded to the color from maroon to brown.
The drying was done in ambient conditions under shade, during three days, for which they were placed on a cloth and constantly removed to obtain a homogeneous drying. The process of extracting the seeds was done manually from fruits selected according to the best phytosanitary condition and of greater length and width. The extracted seeds were dried, by a process similar to the fruits in environmental conditions on a laboratory table. The seeds were partially cleaned, by means of a manual procedure, eliminating the remains of larger fruits.
The ISTA Standards (2016) were used to determine the quality of the seeds in terms of purity, weight of 1,000 seeds, humidity content and germination power.
Three factors were studied: type of containers, storage medium and time. The levels by factors were:
Types of containers: FT- translucent cover; FC- dark plastic cover; CT- translucent glass; CC- amber glass.
Storage medium: N- natural to the environment and R- refrigerated 6-8 °C.
Time. First test: T1- one month; T2- two months; T3- three months; T4- four months; T5- five months and T6- six months.
Second trial T1- for one week; T2- for two weeks; T3- for three weeks and T4- for four weeks.
There were 48 treatments evaluated for the first trial and 32 treatments for the second trial, using an unrestricted randomized design, under laboratory conditions.
The observations on germination were made during 40 days, at 10:00 a.m.
Germination power (Equation 1).
Germinative vigor (Equation 2).
Where:
VM |
maximum or top value that is presented between the values product of the division of the accumulated percentage of germination and the number of days that it took to obtain it |
GDM |
is the average daily germination, calculated as the ratio between the final percentage of germination (PG) and the number of days it took to reach that value |
The assumptions for carrying out the analysis of trifactor variance were verified, which were not fulfilled, and a non-parametric Kruskal-Wallis test was carried out.
Results
The collected seed showed a purity of 75.8 %, which can be given by the type of fruit that is a dehiscent capsule, reducing impurities from the process of drying the fruit and at the time of extraction. It was obtained a weight of 0.315 g for 1,000 seeds of C. pubescens, while the percentage of humidity content of the seeds was 13.6 %. The germinative power was very low, 12 %, the beginning of germination at 29 days and culminated at 36 days, while the value of 0.43 for germinative vigor was negligible.
Storage tests
In the first germination test on C. pubescens seeds, after one month of storage, zero percentage of germination was obtained, for the environments and types of containers used.
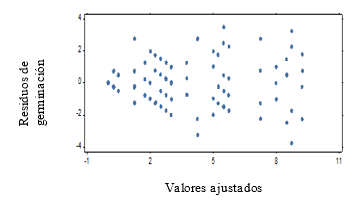
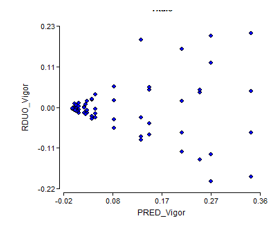
For the second test with weekly evaluation periods it is obtained that, the assumptions of homogeneity and normality for the results of the germination power are not fulfilled (Figure 1 and Figure 2), which shows a fan shape in the distribution of the residuals of germination.
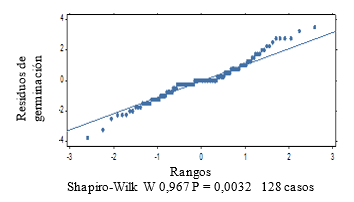
The normality graph of the germination power data (Figure 2), plus the results of the Shapiro-Wilk test at P < 0.05, indicate the non-fulfillment of the normality assumption. The Kruskal-Wallis test (Table 1) shows significant differences between treatments (P = 0.002).
Table 1 - Germination power of Treatments
| Treatments | Media | Treatments | Media |
| FTRT1 | 9,25 a | FTNT4 | 0,00 j |
| FTNT1 | 8,75 ab | CCRT4 | 0,00 j |
| CTRT1 | 8,50 abc | CTNT4 | 0,00 j |
| CCNT1 | 8,00 abc | FCNT4 | 0,00 j |
| CTNT1 | 7,25 abcd | FCRT4 | 0,00 j |
Types of containers: CT- translucent glass; CC- amber glass; FT- translucent plastic cover; FC- black plastic cover; R- refrigeration; N- ambient; T1- one week and T4- four weeks Different letters in the rows and columns indicate significant differences for P < 0.05
The treatment with transparent sleeves in refrigeration for one week, with 9.25 % germination, was the best treatment (Table 1), while those with the worst behavior correspond to the treatments of week four, regardless of the medium and the type of container, since they show zero percentages of germination.
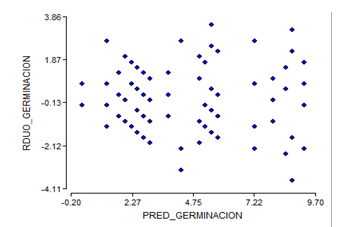
By eliminating the treatments of week four of storage, the assumptions of homogeneity and normality for the results of the germination power are not fulfilled (Figure 3 and Figure 4), which shows a fan shape in the distribution of the residuals of germination.
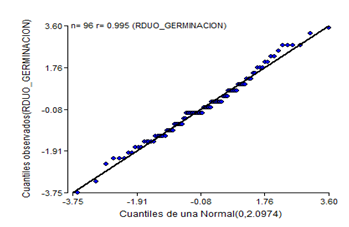
Fig. 4. - Normal probability diagram of the germination power of C. pubescens seeds for three weeks of storage
The normality graph of the germination power data at three weeks (Figure 4), plus the results of the Shapiro- Wilk test at P < 0.05, indicate that the normality assumption was not fulfilled.
The Kruskal-Wallis test shows significant statistical differences between the treatments for the three weeks of storage with respect to the variable germination power. The mean comparison test (Table 2) shows differences between treatments. The treatment of transparent sheath in refrigeration is ratified at week one within the best group, to which the treatments of transparent sheath in natural medium and translucent glass in refrigeration are also incorporated, both also for the first week.
Table 2 - Power and germinative vigor of treatments for three weeks' storage
| Treatments | Germinative power germinativo (%) | Germinative vigor germinativo | Treatments | Germinative power germinativo (%) | Vigor germinativo | Treatments | Poder germinativo (%) | Germinative vigor germinativo |
| FTRT1 | 9,25 a | 0,35 a | FTNT2 | 0,03 de | 0,03 de | FTRT3 | 2,25 cdef | 0,01 e |
| FTNT1 | 8,75 a | 0,27 ab | FCRT1 | 0,08 cde | 0,08 cde | CCRT2 | 2,25 cdefg | 0,01 e |
| CTRT1 | 8,50 a | 0,25 ab | CTNT2 | 0,04 de | 0,04 de | FCRT3 | 2,00 defg | 0,01 e |
| CCNT1 | 8,00 ab | 0,15 bcde | CCNT2 | 0,04 de | 0,01 e | FTNT3 | 1,75 defg | 0,001 e |
| CTNT1 | 7,25 abc | 0,21 abcd | CTRT3 | 3,00 bcdef | 0,01 e | CCRT3 | 1,25 efg | 0,001 e |
| CTRT2 | 5,75 abcd | 0,04 de | FCRT2 | 3,00 bcdef | 0,01 e | CTNT3 | 1,25 efg | 0,01 e |
| FTRT2 | 5,50 abcd | 0,03 de | FCNT1 | 0,02 e | 0,02 e | FCNT2 | 1,25 fg | 0,001 e |
| CCRT1 | 5,50 abcd | 0,13 bcde | CCNT3 | 2,75 cdef | 0.01 e | FCNT3 | 0,025 g | 0,001 e |
Types of Legend: Containers: CT- translucent glass; CC- amber glass; FT- translucent plastic cover; FC- black plastic cover; R- refrigeration; N- ambient; T1- one week; T2- two weeks; T4- three weeks; T4- four weeks Kruskal Wallis at P <0.01. Different letters in the rows and columns indicate significant differences for P < 0.05
The results of the normality and homogeneity tests for germinative vigour showed a similar tendency to germination power. Both tests are not fulfilled (Figure 5 and Figure 6), where it is observed with typical cone for homogeneity and normality ratifies its non fulfilment according to the Shapiro-Wilk test at P < 0.01.

Fig. 6. - Normal probability diagram of germinative vigor of C. pubescens seeds for three weeks of storage
The results of the comparison of means for germinative vigor (Table 2) show that there is no biunivocal relationship between the order of treatments with respect to germinative power. The best treatment is the refrigerated translucent sheath treatment after one week, but the group in which the treatments are located changes with respect to the grouping of the averages for germination power.

Different letters indicate significant differences for P < 0.05
Fig. 7. - Power behavior and germinative vigor of the factors under study
When comparing the averages of the study factors for germination power and vigor, Figure 7, it is observed that the translucent containers are superior to the dark colored ones where the transparent cover stands out, while there are no significant differences between the averages and week one reaches a higher average than the rest, being week three the one with the worst behavior.
Discussion
In the experience, 77.1 % purity was obtained in C. officinalis, while (Caraguay et al., 2016), it reached 38.04 %, in the same species. This result is given by the benefit process carried out from the selection of the fruits of greater size and better sanitary state, plus the handling of the seeds from the extraction of the fruits and its initial cleaning, all of which allowed to have a seed with less impurities.
The weight of 1,000 seeds obtained, is greater than that posed (Campos et al., 2014) of 0.024 g. We agree with these authors that this behavior could be one of the disadvantages of germination, because when the seeds are smaller they have minimum reserves (Pascualides and Ateca, 2013; Alvarado et al., 2015; Rodríguez and Pompa, 2016; Ruíz et al., 2018), which can make use of it quickly after the harvest, so it contributes little to the growth of the new plant. This constitutes a risk for the non-occurrence of germination, since this so complex process can be initiated from the phase of imbibition, without necessarily occurring the activation of the synthesis and degradation, leading to the division and cellular elongation, so that the rupture of the seminal cover by the embryo occurs.
The result of humidity in the seeds, 13.6 %, is lower than that found by (Campos et al., 2014), with a value of 16.67 % in the same species. The initial humidity content can influence the period of imbibition of the seed (Hernández et al., 2018), which implies a greater speed in the absorption of water for seeds with lower humidity content. This greater speed of absorption at the beginning of the imbibition does not imply a superior behavior in the germination of the seeds (Vargas et al., 2015), that when studying two contents of humidity of the seed in four species (S. saman, 11.5 and 6.1 %; P. dulce, 13.8 and 5.5 %; J. caucana, 8.4 and 3.5 %; T. rosea, 8.3 and 3.6 %) does not obtain significant differences for the germination between the levels of initial humidity of the seeds for each species. On the other hand (Lines et al., 2006), they evaluated five moisture contents (4.8 %, 10.5 %, 21.3 %, 26.3 % and 40.3 %) and found that the seed germinates in less time and greater percentage with the highest humidity content and that the decrease in the germination percentage is proportional to the decrease in the humidity content.
A greater time of drying of the seeds in reason of obtaining a smaller content of humidity in these, can affect its germinative capacity since, the absorption of humidity by the seeds to reach its hygroscopic balance during the process of imbibition, causes the deterioration of the plasmatic membrane of the seeds and diminishes its physiological quality (Crivelari et al., 2019).
The behavior of the germinative power, 12 % was very low, which can be given by different causes, such as: undeveloped embryos, dead embryos, very small seeds and low germinative reserve, among others. The seeds of C. pubescens present difficulties in their germination, (Campos et al., 2014), since it is directly influenced by the physiological maturation of the seeds. A cause to be considered would also be the level of humidity of the seeds once the drying process is done, since a low percentage of humidity implies that the seed has a negative matric potential, so it tends to soak very quickly (phase I), regardless of whether the seed is dormant or viable (Matilla, 2008).
Small seeds with low reserves and low humidity, make a rapid absorption of water, which causes temporary alterations in the differential permeability of the membranes of the seed and, therefore, a loss to the surrounding environment of solutes and different metabolites of low molecular weight (sugars, organic acids, ions, amino acids, peptides, etc.), corroborated in the results of (Ribeiro et al., 2015). The results make it possible to assume as low the metabolic activation of the seeds, which prevents them from developing phase II (plateau), a period of delayed water absorption, without reaching the activation of the embryo. Dead and dormant seeds maintain this level of typical hydration of phase II, but unlike seeds germinating they do not enter phase III, which is associated with the protrusion of the radicle. Everything agrees with what was concluded by (Marler, 2019), since the speed of imbibition during the physical phase does not necessarily correlate directly with the speed of final germination.
In imbibition, seeds are transformed from an inactive dry state (without translation) to a fully active metabolic state, and selectively translate subsets of these stored RNAm. Therefore, the seeds provide a unique on/off switch (Sajeev et al., 2019), regulated by the development for translation. The results of the very low germination power of C. pubescens seeds in the study suggest that their activation could be reduced in seeds that failed to germinate, even when they developed phase I imbibition.
In relation to the maximum time of germination, 36 days, do not coincide with that obtained by (Bargali and Singh, 2007) which was 22 days, nor with Campos et al., (2014), Cinchona sp. from the town of La Cascarilla-Jaén, with original soil substrates, which fluctuated between 12 and 24 days, independently of the origin of the different places of the mentioned town.
The germinative vigour was negligible, 0.43, in accordance with the low germination power and the duration of germination. Those species that distribute the energy they devote to fructification in producing a large number of small seeds, as is the case of C. pubences, this capacity to be widely distributed because it has greater opportunities for the seeds to find a favorable place to grow (Rossetto et al., 2000). Its small size contributes little to the growth of the new plant, which has a high probability of dying because it depends quickly on the resources available in the environment. In addition, these plants are sensitive to damage from biotic and abiotic agents, and their survival is minimal, which is compensated by the large number of seeds that individuals of this species produce.
Storage tests
The non-germination of the seeds after one month of storage coincides with what was stated by Acosta (1945), referring to the fact that when the seeds are fresh they germinate between a period of 11 and 20 days and if they are old, depending on the storage period, the germination percentage decreases, therefore the seeds of this species when they are subjected to storage for more than one month, lose their viability.
The results show that the time factor influences the germination since, the seeds of C. pubescens when being submitted to each one of the treatments of conservation in times from 1 to 4 weeks, the percentage of germination diminishes as the time of conservation is increased until arriving at a value of zero to the fourth week, independently of the means of storage and the type of used package.
The treatments under refrigeration conditions for the different types of containers in the first week of storage showed a better trend in the germination behavior Ortiz et al., (2004) highlight the advantage of air-conditioned chambers with domestic air conditioning, confirming the favorable effect of low temperatures in seed storage. On the other hand, Ruíz et al., (2017), Valverde et al., (2019), ratify that the refrigerated treatments showed significance with respect to the non-refrigerated treatments.
When studying the storage of Dypsis lutescens seeds, Doria et al. (2012), found that the best container was black polyethylene followed by translucent plastic bottles, while the best treatments were black polyethylene in refrigeration and environmental conditions. These results differ from those obtained in this research because the translucent polyethylene sleeves were the best behaved, but they coincide as they are the best containers in both storage conditions.
In relation to the light requirement, most tree species behave as indifferent (Flores et al., 2017), germinating under both light and dark conditions. Based on the fact that, ecologically, the perception of light by the seed can act as an indicator of the amount of light available to the seedling, it can indicate planting depth, canopy shading and soil disturbance. This behavior is supported by the fact that the presence or absence of light indicates to the seeds if they are close to the surface or buried, on the other hand the red/distant red ratio (R/RL) is an indicator of the presence and size of forest clearings, that is to say, of the density of the canopy according to what Escobar and Cardoso (2015) have exposed. In the experience, four of the five treatments with the best behavior have the characteristic of a translucent type of container, which allows us to assume that the seeds of C. pubencens could better manifest their germination, with certain levels of presence of light during storage, either artificial or natural in the soil seed bank.
By using airtight storage, containers that prevent air and humidity from entering the product. In these conditions, the breathing of the seed and the insects (when there are any) deplete the existing oxygen, causing the death of the latter and the reduction of the activity of the seed, so the storage can last a long time without deterioration.
When opening the containers to extract the seeds at the time of the germination assemblies, a gaseous exchange with the local environment is carried out. Once the container is closed, an exchange of humidity between the seeds and the environment inside the container is restored, which generally causes an increase in the humidity content of the seeds. This aspect is more relevant for storage in environmental conditions, which can accelerate the deterioration of the seeds, unlike the cold environment since the humidity in the microenvironment of the container is lower, since the relative humidity in the refrigerator is also lower (Ruíz et al., 2017).
The germinative vigour of the treatments shows a similar tendency to that of the germinative power, but it is distinguished that there are some increases for some cases, which is given by the reduction of the number of days in manifesting the maximum accumulated germinative power. In experience, values of less than one to zero are obtained, while Campos et al. (2014) achieved values from zero to 13.5 of germinative vigor.
Conclusions
The quality of the seeds of C. pubescens is low in relation to the percentage of germination, of low weight and average physical purity.
The germination of the seeds of C. pubescens does not occur after a month of storage, with a significant reduction from the first week, in both transparent covers and packaging is the best option, without any difference between the means of refrigeration and natural environment.
Referencias bibliográficas
ACOSTA SOLÍS, M., 1945. Botánica de las Cinchonas. Flora, vol. 6, no. 15-16, pp. 29-55. [ Links ]
ALVARADO VÁZQUEZ, M.A., FOROUGHBAKHCH, R., RÄHIM, FOROUGHBAKHCH., GUZMÁN LUCIO, M.A., ROCHA ESTRADA, A., HERNÁNDEZ PIÑERO, J.L., CÁRDENAS ÁVILA, M.L. y SOTO GARCÍA, B.M., 2015. Efecto de la madurez del fruto, peso de la semilla y tiempo de almacenamiento en la viabilidad y germinación de la semilla de candelilla (Euphorbia antisiphylitica Zucc.). Phyton [en línea], vol. 84, pp. 70-79. [Consulta: 15/02/2020]. Disponible en: Disponible en: https://www.researchgate.net/publication/317532447_Efecto_de_la_madurez_del_fruto_peso_de_la_semilla_y_tiempo_de_almacenamiento_en_la_viabilidad_y_germinacion_de_la_semilla_de_candelilla_Euphorbia_antisiphylitica_Zucc . [ Links ]
ANDERSSON, L; TAYLOR, C. 1994. Rubiaceae cinchoneae coptosapelteae. En: HARLING, G., ANDERSSON, L., (Eds), Flora of Ecuador no 50. Council for nordic publications in Botany. Museo Botánico. pp.114 . [ Links ]
BARGALI, K. y SINGH, S.P., 2007. Germination behaviour of some leguminous and actinorhizal plants of Himalaya: Effect of temperature and medium. Tropical Ecology [en línea], vol. 48, no. 1, pp. 99-105. [Consulta: 23/11/2019]. ISSN 0564-3295. Disponible en: Disponible en: https://www.researchgate.net/publication/238087379_Germination_behaviour_of_some_leguminous_and_actinorhizal_plants_of_Himalaya_Effect_of_temperature_and_medium . [ Links ]
BLANCO VALDÉS, Y., DURAÑONA, H. y ACOSTA ROCA, R., 2016. Efecto de la temperatura y la humedad en la conservación de granos de maíz en silos metálicos refrigerados. Cultivos Tropicales [en línea], vol. 37, no. 4, pp. 105-114. [Consulta: 21/09/2020]. ISSN 0258-5936. DOI 10.13140/RG.2.2.13900.21127. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-59362016000400010&lng=es&nrm=iso&tlng=es . [ Links ]
BUDDENHAGEN, C.E., RENTERIA, J.L., GARDENER, M., WILKINSON, S.R., SORIA, M., YÁNEZ, P., TYE, A. y VALLE, R., 2004. The Control of a Highly Invasive Tree Cinchona pubescens in Galapagos1. container-title: Weed Technology, Weed Technology [en línea], vol. 18, no. sp1, pp. 1194-1202. [Consulta: 17/03/2020]. ISSN 0890-037X, 1550-2740. DOI 10.1614/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2. Disponible en: Disponible en: https://bioone.org/journals/Weed-Technology/volume-18/issue-sp1/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2/The-Control-of-a-Highly-Invasive-Tree-Cinchona-pubescens-in/10.1614/0890-037X(2004)018[1194:TCOAHI]2.0.CO;2.full . [ Links ]
CAMPOS RUÍZ, J., REBAZA DE CHICO, L.C. y CHICO RUÍZ, J., 2014. Efecto del ácido giberélico, nitrato de potasio y agua de coco en la germinación de semillas de quina, Cinchona pubescens. Revista REBIOLEST [en línea], vol. 2, no. 1, pp. 5-15. [Consulta: 27/03/2020]. Disponible en: Disponible en: https://revistas.unitru.edu.pe/index.php/ECCBB/article/view/637 . [ Links ]
CARAGUAY YAGUANA, K.A., ERAS GUAMAN, V.H., GONZÁLEZ ZARUMA, D., MORENO SERRANO, J., MINCHALA PATIÑO, J., YAGUANA ARÉVALO, M. y VALAREZO ORTEGA, C., 2016. Potencial reproductivo y análisis de calidad de semillas de Cinchona officinalis L., provenientes de relictos boscosos en la provincia de Loja-Ecuador. Revista Investigaciones Altoandinas [en línea], vol. 18, no. 3, pp. 271-280. ISSN 2306-8582. [Consulta: 18/02/2020] Disponible en: Disponible en: https://dialnet.unirioja.es/servlet/articulo?codigo=5645611 . [ Links ]
CEBALLOS, A.J. y LOPEZ, J.A., 2007. Conservación de la calidad de semillas forestales nativas en almacenamiento. En: Accepted: 2012-04-03T21:33:57Z, Cenicafé [en línea], vol. 58, no. 4, pp. 265-292. [Consulta: 18/02/2020]. ISSN 0120-0275. Disponible en: Disponible en: https://biblioteca.cenicafe.org/handle/10778/116 . [ Links ]
CRIVELARI DA COSTA, P.M., BIANCHINI, A., CANEPPELE, C., AZEVEDO, P.H., SILVA, A.L. da, AZEVEDO DOS SANTOS, M. y XAVIER PEREIRA, P.S., 2019. Moisture Content and Packaging Condition on the germination of Amaranth BRS Alegria Seeds. Journal of Experimental Agriculture International [en línea], vol. 38, no. 4, pp. 1-8. [Consulta: 27/03/2020]. ISSN 2457-0591. Disponible en: Disponible en: https://www.researchgate.net/publication/334218032_Moisture_Content_and_Packaging_Condition_on_the_Germination_of_Amaranth_BRS_Alegria_Seeds . [ Links ]
DORIA GONZÁLEZ, J., BENÍTEZ FERNÁNDEZ, B. y SOTO, F., 2012. Influencia de diferentes métodos de conservación en la germinación de semillas de palma areca (Dypsis lutescens, H. Wendel). Cultivos Tropicales [en línea], vol. 33, no. 2, pp. 56-60. [Consulta: 28/07/2020]. ISSN 1819-4087. Disponible en: Disponible en: https://www.researchgate.net/publication/262465901_Influencia_de_diferentes_metodos_de_conservacion_en_la_germinacion_de_semillas_de_palma_areca_Dypsis_lutescens_H_Wendel . [ Links ]
DORIA, J., 2010. Generalidades sobre las semillas: su producción, conservación y almacenamiento. Cultivos Tropicales [en línea], vol. 31, no. 1. [Consulta: 20/08/2020]. ISSN 0258-5936. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S0258-5936201 00001 00011&lng=es&nrm=iso&tlng=es . [ Links ]
ESCOBAR, D.F. y CARDOSO, V.J.M., 2015. Germinación y latencia de semillas de miconia chartacea (Melastomataceae), en respuesta a luz, temperatura y hormonas vegetales. En: Accepted: 2018-12-11T16:41:40Z, Revista de Biologia Tropical [en línea], vol. 63, no. 4, pp. 1169-1184. [Consulta: 20/08/2020]. ISSN 2215-2075. DOI 10.15517/rbt.v63i4.17955. Disponible en: Disponible en: https://repositorio.unesp.br/handle/11449/168533 . [ Links ]
FLORES, P., POGGI, D., GARCÍA, S., CATRARO, M. y GARIGLIO, N., 2017. Ruptura de la dormición y exigencias de luz para la germinación de semillas de Juglans nigra. Revista FAVE-Ciencias Agrarias [en línea], vol. 16, no. 2, pp. 33-46. [Consulta: 27/07/2020]. ISSN 2346-9129. DOI 10.14409/fa.v16i2.7018. Disponible en: Disponible en: https://www.researchgate.net/publication/322086032_Ruptura_de_la_dormicion_y_exigencias_de_luz_para_la_germinacion_de_semillas_de_Juglans_nigra . [ Links ]
GARMENDÍA, S.A., 2005. El árbol de la quina (Cinchona spp.): distribución, caracterización de su hábitat y arquitectura [en línea]. Ecuador: Universidad Técnica Particular de Loja. ISBN 978-9978-09-574-4. [Consulta: 27/03/2020]. Disponible en: Disponible en: https://books.google.com.cu/books/about/El_%C3%A1rbol_de_la_quina_Cinchona_spp.html?id=lnMgPQAACAAJ&redir_esc=y . [ Links ]
GÓMEZ SILVERA, A., BERAUN MACEDO, L.A., GÓMEZ RENGIFO, O.J. y LLATAS DUCEP, E., 2016. Procesos de regeneración natural de la quina o cascarilla (Cinchona spp.): en los bosques de neblina del distrito de Kañaris, región Lambayeque. INIA. Estación Experimental Agraria Vista Florida-Lambayeque [en línea], [Consulta: 20/08/2020]. Disponible en: Disponible en: http://repositorio.inia.gob.pe/bitstream/inia/572/1/Gomez-procesos_reg.pdf . [ Links ]
GÜNTER S., B., STIMM, Y. M. WEBER. 2004. Silvicultural contributions towards sustainable management and conservation of forest genetic resources in Southern Ecuador. Lyonia vol. 6, 1: 75-91. [Consulta: 22/11/2019]. Disponible en: Disponible en: http://www.lyonia.org/articles/rbussmann/article_307/pdf /article.pdf [ Links ]
HERNÁNDEZ ANGUIANO, L.A., LÓPEZ UPTON, J., RAMÍREZ HERRERA, C., ROMERO MANZANARES, A., HERNÁNDEZ ANGUIANO, L.A., LÓPEZ UPTON, J., RAMÍREZ HERRERA, C. y ROMERO MANZANARES, A., 2018. Variación en germinación y vigor de semillas de Pinus cembroides y Pinus orizabensis. Agrociencia [en línea], vol. 52, no. 8, pp. 1161-1178. [Consulta: 17/01/2020]. ISSN 1405-3195. Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1405-31952018000801161&lng=es&nrm=iso&tlng=es . [ Links ]
HERRERA, J., LINES, K. y VÁSQUEZ, W., 2006. Estudio de la germinación y la conservación de semillas de cedro maría (Calophyllum brasiliense). Revista Tecnología en Marcha [en línea], vol. 19, no. 1. [Consulta:27/03/2020]. Disponible en: Disponible en: https://www.researchgate.net/publication/277198452_Estudio_de_la_germinacion_y_la_conservacion_de_semillas_de_cedro_maria_Calophyllum_brasiliense . [ Links ]
INTERNATIONAL SEED TESTING ASSOCIATION (ISTA). 2016. Reglas Internacionales para el Análisis de las Semillas. Zürichstr. 50, CH-8303 Bassersdorf, Suiza. Versión en español. [Consulta: 22/10/2018]. Disponible en: Disponible en: https://vri.umayor.cl/images/ISTA_Rules_2016_Spanish.pdf [ Links ]
JÄGER, H., 2015. Biology and Impacts of Pacific Island Invasive Species. 11. Cinchona pubescens (Red Quinine Tree) (Rubiaceae). Pacific Science [en línea], vol. 69, no. 2, pp. 133-154. [Consulta: 20/07/2020]. DOI 10.2984/69.2.1. Disponible en: Disponible en: https://www.researchgate.net/publication/281129770_Biology_and_Impacts_of_Pacific_Island_Invasive_Species_11_Cinchona_pubescens_Red_Quinine_Tree_Rubiaceae . [ Links ]
JÄGER, H. y KOWARIK, I., 2010. Resilience of native plant community following manual control of invasive Cinchona pubescens in Galápagos. Restoration Ecology [en línea], vol. 18, no. 1, pp. 103-112. [Consulta: 22/11/2019]. ISSN 1526-100X. Disponible en: Disponible en: https://www.researchgate.net/publication/227724741_Resilience_of_Native_Plant_Community_Following_Manual_Control_of_Invasive_Cinchona_pubescens_in_Galapagos . [ Links ]
MAHECHA VEGA, G., OVALLE ESCOBAR, A., CAMELO SALAMANCA, D., ROZO FERNÁNDEZ, A. y BARRERO BARRERO, D., 2016. Vegetación del territorio CAR: 450 especies de sus llanuras y montañas [en línea]. Colombia: Corporación Autónoma Regional de Cundinamarca-CAR. ISBN 978-958-8188-06-5. [Consulta: 27/03/2020]. Disponible en: Disponible en: https://books.google.com.cu/books/about/Vegetaci%C3%B3n_del_territorio_CAR.html?id=wKvitgAACAAJ&redir_esc=y . [ Links ]
MARLER, T., 2019. Temperature and Imbibition Influence Serianthes Seed Germination Behavior. Plants [en línea], vol. 8, no. 4, pp. 107. DOI 10.3390/plants8040107. Disponible en: https://www.semanticscholar.org/paper/Temperature-and-Imbibition-Influence-Serianthes-Marler/a158bbae75e4742d13f6f42c427f32fe31c82031. [ Links ]
MATILLA, A.J., 2008. Desarrollo y germinación de las semillas. En: AZCÓN BIETO, J. y TALÓN, M. Fundamentos de Fisiología Vegetal [en línea]. S.l.: McGraw Hill, pp. 537-558. [Consulta: 22/11/2019]. Disponible en: Disponible en: https://www.researchgate.net/publication/271512205_Desarrollo_y_germinacion_de_las_semillas . [ Links ]
ORTIZ, R., FÉ, C. de la y PONCE, M., 2004. Evaluacion de metodos de almacenaje de semilla de soya (Glycine max. (L.) Merrill) en condiciones de bajos insumos. Cultivos Tropicales [en línea], vol. 25, no. 3, pp. 49-59. [Consulta: 22/11/2019]. ISSN 0258-5936. Disponible en: Disponible en: https://go.gale.com/ps/i.do?p=IFME&sw=w&issn=02585936&v=2.1&it=r&id=GALE%7CA174373711&sid=googleScholar&linkaccess=abs . [ Links ]
PASCUALIDES, A.L. y ATECA, N.S., 2013. Germinación y vigor de morfotipos de semillas de Crotalaria juncea L. (Fabaceae). Phyton [en línea], vol. 82, pp. 313-319. [Consulta: 15/06/2020]. ISSN 0031-9457. Disponible en: Disponible en: https://www.semanticscholar.org/paper/Germinaci%C3%B3n-y-vigor-de-morfotipos-de-semillas-de-L.-Pascualides-Ateca/ccca72612dfc21d522afd0e73e98d6eeede69909 . [ Links ]
PRADO, L., SAMANIEGO, C. y UGARTE GUERRA, J., 2010. Estudio de las cadenas de abastecimiento de germoplasma forestal en Ecuador [en línea]. Perú: ASB-World Agroforestry Centre. [Consulta: 27/03/2020]. Disponible en: Disponible en: http://old.worldagroforestry.org/downloads/Publications/PDFS/WP16813.pdf . [ Links ]
RIBEIRO, P.R., WILLEMS, L.A.J., MUDDE, E., FERNANDEZ, L.G., CASTRO, R.D. de, LIGTERINK, W. y HILHORST, H.W.M., 2015. Metabolite profiling of the oilseed crop Ricinus communis during early seed imbibition reveals a specific metabolic signature in response to temperature. Industrial Crops and Products [ en línea], vol. 67, pp. 305-309. [Consulta: 18/02/2020]. ISSN 0926-6690. DOI 10.1016/j.indcrop.2015.01.067. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S0926669015000692 . [ Links ]
RODRÍGUEZ TREJO, D.A. y POMPA GARCÍA, M., 2016. Tamaño, color de nuez y sombra afectan la germinación de Quercus desertícola. Madera y bosques [en línea], vol. 22, no. 2, pp. 67-75. [Consulta: 15/07/2020]. ISSN 1405-0471. DOI 10.21829/myb.2016.2221325. Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S1405-04712016000200067&lng=es&nrm=iso&tlng=es . [ Links ]
ROSSETTO, C., CONEGLIAN, R.C.C., NAKAGAWA, J., SHIMIZU, M.K. y MARÍN, B., 2000. Germinação de sementes de maracujá-doce (Passiflora alata Dryand.) em função de tratamento pré-germinativo. Revista Brasileira de Sementes, vol. 22, no. 1, pp. 247-252. [Consulta: 27/03/2020]. Disponible en: DOI 10.17801/0101-3122/. [ Links ]
RUÍZ PÉREZ, A., ARAMÉNDIZ TATIS, H. y CARDONA AYALA, C., 2017. Efecto del almacenamiento en la calidad fisiológica de semilla de moringa (Moringa oleifera Lam.). Revista U.D.C.A Actualidad & Divulgación Científica [en línea], vol. 20, no. 1, pp. 79-89. [Consulta: 21/01/2020]. ISSN 2619-2551. DOI 10.31910/rudca.v20.n1.2017.65. Disponible en: Disponible en: https://revistas.udca.edu.co/index.php/ruadc/article/view/65 . [ Links ]
RUÍZ SÁNCHEZ, M., MUÑOZ HERNÁNDEZ, Y., GUZMÁN, D., VELÁZQUEZ RODRÍGUEZ, R., DÍAZ LÓPEZ, G. S., MARTINEZ, A. Y., & ALMEIDA, F. M. 2018. Efecto del calibre semilla (masa) en la germinación del sorgo.Cultivos Tropicales, vol 39 no. 4, 51-59. [Consulta: 27/03/2019]. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0258-59362018000400007&lng=es&tlng=es . [ Links ]
SAJEEV, N., BAI, B. y BENTSINK, L., 2019. Seeds: A Unique System to Study Translational Regulation. Trends in Plant Science [en línea], vol. 24, no. 6, pp. 487-495. [Consulta: 22/11/2019]. ISSN 1360-1385. DOI 10.1016/j.tplants.2019.03.011. Disponible en: Disponible en: https://research.wur.nl/en/publications/seeds-a-unique-system-to-study-translational-regulation . [ Links ]
ULLOA ULLOA, C. y JORGENSEN, P.M., 1995. Árboles y Arbustos de los Andes del Ecuador [en línea]. Ecuador: Ediciones Abya-Yala. [Consulta: 21/01/2020]. ISBN 978-9978-04-157-4. Disponible en: Disponible en: https://isbn.cloud/9789978041574/arboles-y-arbustos-de-los-andes-del-ecuador/ . [ Links ]
VALVERDE RODRÍGUEZ, K., MORALES, C.O. y GARCÍA, E.G., 2019. Germinación de semillas de Crescentia alata (Bignoniaceae) en distintas condiciones de temperatura, luminosidad y almacenamient. Biología Tropical [en línea], vol. 67, no. 2, pp. 120-131. [Consulta: 27/03/2020]. ISSN 0034-7744. Disponible en: Disponible en: https://revistas.ucr.ac.cr/index.php/rbt/article/download/37211/37857/ . [ Links ]
VARGAS FIGUEROA, J.A., DUQUE PALACIO, O.L. y TORRES GONZÁLEZ, A.M., 2015. Germinación de semillas de cuatro especies arbóreas del bosque seco tropical del Valle del Cauca, Colombia. Revista de Biología Tropical [en línea], vol. 63, no. 1, pp. 249-261. [Consulta: 18/02/2020]. ISSN 0034-7744. Disponible en: Disponible en: http://www.scielo.sa.cr/scielo.php?script=sci_abstract&pid=S0034-774420150001 00020&lng=en&nrm=iso&tlng=es . [ Links ]
Received: August 02, 2020; Accepted: September 20, 2020











 texto en
texto en