Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Ciencias Forestales
versión On-line ISSN 2310-3469
Rev cubana ciencias forestales vol.8 no.3 Pinar del Río sept.-dic. 2020 Epub 11-Sep-2020
Original article
Characterization of colonization by micorhizae in Retrophyllum rospigliossi Pilger in the Huamantanga forest, Peru
1Universidad Nacional de Cajamarca. Perú.
2Universidad de La Habana. La Habana, Cuba.
Podocarpacea are the only native conifers of Peru, a country that contains one of the most important South American populations of one of its member species: Retrophyllum rospigliossi Pilger, which lives in the rainforests, where it is only preserved in small relics; due to this and the difficulties for reproduction and conservation in-situ is considered a vulnerable species. Among the microorganisms of the soil, the mycorrhizal fungi stand out for their symbiotic activity by allowing efficient nutrition, being beneficial for many species of trees, providing them with the necessary conditions for their survival. The objective of this work was to characterize the mycorrhizal colonization of Retrophyllum rospigliossi Pilger in the categories of saplings, latizal and fustal, and for this purpose, samples were collected in five georeferenced plots. The roots were colored with trypane blue for the observation of hyphae, vesicles, and shrubs. Soil samples were sifted for spore count. Mycorrhizal structures were found in all three categories, with significant differences for spores and vesicles. The behavior of these per gram of soil was variable (hyphae: 39.2 % to 53 %, spores 30.8 % to 57.6 %, vesicles: 35 % to 51.4 %, shrubs: 0 to 2 %). Colonization of roots ranged from 85 % to 96.2 %. Three mycorrhizal genera were identified: Glomus, Entrophospora and Acaulospora. It is concluded that, for a vegetative propagation of woody species, it is necessary to consider the use of arbuscular mycorrhizae to guarantee an adequate nutrition.
Key words: Arbuscular mycorrhizae fungus; Saplings; Latizal; Fustal.
Introduction
The need to redesign the existing ecosystem models, so that they allow the consequences of human activities to be understood within the framework of the complexity that really characterizes ecosystems, is a topic that has recently received much attention. In this sense, it is the diversity of interactions that is first lost in response to global change, long before changes in biodiversity are even detected (Ochoa, 2017).
The mountain cloud forests of the tropics are considered very fragile ecosystems, because they play a hydrological and ecological role. Armenteras et al., (2015) and the Food and Agriculture Organization of the United Nations (FAO, 2016), point out that the conversion of native forests to agricultural areas is established as the factor with the greatest impact on most terrestrial ecosystems, because it reduces the area of natural habitats, constituting a threat to biodiversity. Likewise, the removal of natural vegetation cover and the excessive use of natural resources have intensified soil desertification processes.
Mountain agriculture has managed to survive by maintaining ancestral practices that correspond to agro-ecological approaches, which has made it possible to generate sustainable agriculture, due to the low dependence on external inputs and the mitigation of the effects caused by the Green Revolution on the environment, circumstances that have an impact on the conservation of soil, water and biodiversity (Acuña and Marchant, 2016). Agro-ecological techniques ensure a sustainable use of natural resources, care of the environment and people, allowing to guarantee the feeding of each family in the long term. One of the practices framed within this context is the use of a great diversity of microorganisms, among which are fungi and bacteria, mixed in different organic substrates to be used in agriculture and livestock (Peña et al., 2019).
Among the microorganisms, mycorrhizae stand out, which are mutualistic associations between soil fungi and plant roots. In this symbiosis, the fungus covers its carbon demands and increases the absorption of water and minerals in the plant, taking mainly elements of slow diffusion such as phosphorus, zinc and copper (Bonilla and Alarcón, 2015; Medina-García, 2016; Pérez et al., 2019), favoring its growth and contributing to the structuring of plant communities, sustainability, functionality and maintenance of natural ecosystems, including degraded ones, being useful for nature and man (Garzón, 2016; Bañuelos et al., 2017; Lattuada et al., 2019).
The Huamantanga forest, located in Jaen, Peru, is very diverse in its type of vegetation, flora and fauna, and like all forests, it is also exposed to logging and land use changes. Among the most important and emblematic species for the inhabitants, due to its wood potential, is Retrophyllum rospigliossi Pilger, commonly known as "romerillo"; however, its natural regeneration is scarce due to the fact that a high percentage of seeds are attacked by insects (beetles), resulting in a low existence of seed trees; On the other hand, there is an alteration of its natural habitat due to the extraction of wood of the high zones of Jaén and in spite of to have carried out a vegetative propagation, the results are minimum, in the same way it is observed in a type of sexual reproduction.
The above-mentioned problem, together with the lack of a forest management protocol, means that R. rospigliossi Pilger cannot be used in a reforestation program, despite its high economic value. Hence the importance of knowing the characteristics of the mycorrhizal association of this plant species in its habitat, since they are precisely: the presence of arbuscular mycorrhizal fungi (AMF) and the level of mycorrhization, two of the variables that are taken into account for the development of cultural techniques in reforestation programs. The aim of this research is to characterize mycorrhizal colonization in Retrophyllum rospigliossi Pilger in the categories of saplings, latizal and fustal. The aim of this research is to characterize mycorrhizal colonization in Retrophyllum rospigliossi Pilger in the categories of saplings, latizal and fustal.
Materials y methods
Field work. Georeference
The sample places were located by satellital image using drone and Agisoft PhotoScan software, in the area, where three categories of development (saplings, latizal and fustal) of R. rospigliosii stands.
There were studied soil samples, which were taken from 10 to 20 cm of deepness R. rospigliosii rhizosphere in the categories saplings (samples until 1m high), latizal (8 to 20 m high and 10 to 30 cm in diameter) and fustal (over 20 m high and 30 to 50 cm in diameter). The studied plots were identified by nearer hamlets´name: Sport Piura (San José del Alto), Nuevo Jerusalén, Guayaquil, San Luis del Nuevo Retiro I and San Luis del Nuevo Retiro II, from district of Jaén, region Cajamarca, Peru (Figure 1).
 Source: Universidad Nacional de Cajamarca.
Source: Universidad Nacional de Cajamarca.Fig. 1. - Placement of localities with the largest incident of three categories of R. rospigliosii Pilger in the Jaén province
For each place and category, there were taken three soil samples of a kg each one, assuring the root presence with a minimum lenght of 1 cm. The samples were processed before 24 hours of collecting, on the contrary, they were kept on freezing to 10 °C.
Lab work
Root staining
The root tinción was done taking into account the methodology proposed by Philips and Hayman (1970) following the way: there were taken the thinest root (smaller than 1 mm of diameter), to facilitate the reagent penetration. The root are placed in small glass plate to be spreaded and avoiding be tight, then it was added KOH to 10% during 30 minutes in order to soften the tissue. Ater that it was placed in a hot bath during 10 minutes. The roots were washed using a fit siever to avoid losses while rinsing. Afterwards the samples were covered with a fresh solution of KOH to 10 % and H2O2 to 10 % mixing them in 1:1 (V/V) proportion during 15 minutes and finally the roots were washed with water. It was added a solution of HCI to 1N during 15 minutes to correct Ph, then HCl without washing was decanted and Trypane blue to 0.05% was added, placing the roots to hot bath for 15 minutes. The colorant was isolated, it was washed with distilled water, it was kept reposing for 12 hours to remove the excess dye and begin observing it using binocular microscopic Olympus CX21.
Quantification of the percentage of colonization
Ten root segments of 1 cm long each were taken and placed in the slide in a parallel way, the observation of the fungal structures was carried out in the compound microscope with the objectives from 10X to 40X, using the technique of determination of colonization by fields of infection, in which sweeps of each root segment were made. The presence of typical structures of the AMF was registered: hyphae, spores, vesicles and bushes.
To determine the percentage of colonization of each sample, the formula proposed by Sieverding (1983) (Equation 1).
The frequency of root colonization was determined by considering the colonized and non colonized segments. It was obtained the total relation of colonized segments (those in which the presence of bushes and vesicles was detected), with respect to the total segments evaluated. The same procedure was used to evaluate the presence of hyphae, vesicles and shrubs, which was expressed as a percentage.
B. Spore density
The samples were worked following the methodology proposed by Sieverding (1983), counting the spores that are observed in the tissues colored with Trypane blue.
C. Arbuscular mycorrhizal fungi spore separation in rhizospheric soils
The soil samples obtained at each sampling point were individually homogenized by removing all thick material (roots, stones and clods); then 100 g samples were taken and placed in a 500 ml beaker, adding 400 ml of distilled water and shaking for 10 minutes. It was filtered through a set of sieves of 4000, 500, 250, 125 and 63 µm (vertically arranged in descending order); the procedure was repeated twice. The content of the 63 µm sieve was poured into a Petri dish which was then dried at room temperature (28 to 30 °C).
D. Centrifugation, decantation and extraction to concentrate the spores
From the dry sifted soil sample, 1 g was weighed and placed in centrifuge tubes, adding 15 ml of 70 % sucrose solution, then centrifuged at 2500 rpm for 10 minutes. Later, the tubes were removed from the centrifuge, taking care not to break the water-sucrose interface. With the help of a 30 cm pipette, the whole surface of the interface was covered, which was filtered in Watman paper N° 4 with the help of a funnel, washed with distilled water to eliminate the sucrose and the spores were observed in the filter paper; this was placed in the compound microscope, a zigzag route was made counting the spores using the 10X and 40X objectives.
E. Identification
For the identification of the spores, their morphological characteristics were analyzed (shape, size, color, surface texture, type of support hyphae and origin); for which they were placed in slides that had a grid in the center with divisions equal to 0.01 mm. They were observed with the 40X objective in the Olympus CX21 binocular composite microscope. The description of the spores was used in the taxonomic identification up to the genus level with the support of keys and descriptions updated by Guerrero and Hodson (1988).
F. Statistical Analysis
The data obtained, once it was proven that they met the assumptions of normality, were applied the analysis of variance (ANOVA) to determine the presence of statistical differences between the percentages of colonization and for each of the percentages of fungal structures observed (hyphae, spores, vesicles, shrubs). In each case, two ANOVAs were performed, one to compare the results between the sampled plots and the other for the comparison between the developmental stages. In the cases where significant differences were detected (P ˂ 0.05), the T test was applied to compare by pairs of elements (plots or developmental stages), from all possible combinations, in order to determine, as the case may be, between which plots or developmental stages such difference was found. In all cases the statistical program SPSS version 26.0 was used.
Results and discussion
The study of the different AMF structures in the rhizosphere of R. rospigliosii, where the microbial biomass is up to 10 times higher than in the soil (Bonilla and Alarcón, 2015), showed the presence of hyphae, spores, vesicles and shrubs in the three categories analyzed.
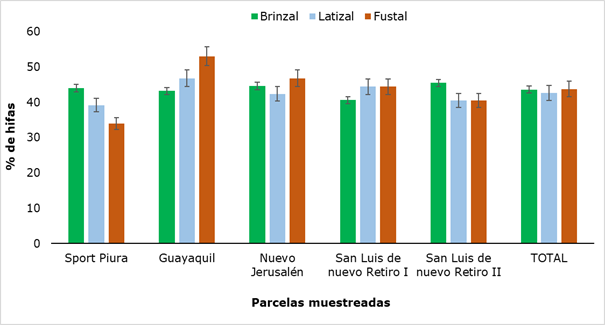
The percentage of hyphae found in the three categories evaluated (Figure 2) showed no significant differences (P ≥ 0,05).
The presence of hyphae varied between 34 % and 53 %, both values obtained in adult trees. Similar results were reported by Gómez (2019), when carrying out the study of arbuscular mycorrhizae in three systems of soil use, determining a percentage of hyphae colonization of 66 % from samples taken at a depth of 10 cm. It is inferred that the presence of hyphae in all sectors and slight variations between places, may be due to soil type, as the Huamantanga forest has a very varied texture, ranging from sandy loam to clay (UNC, 2019).
The presence of hyphae of different thickness found in the roots of the rhizosphere of R. rospigliosii(Figure 3) were classified as Paris and Arum type, with formation of enough curls from which the shrubs originate, and present intercellular hyphae and intracellular shrubs. The results obtained are similar to those of Parra-Rivero et al., (2018) when presenting the types of hyphae present in the colonization by AMF of the radical systems of two tree leguminous plants, observing two simultaneous morphological types: Arum and Paris, being the first one of greater frequency with thick hyphae with H structure, which run parallel to the radical surface in an intercellular way and form intracellular lateral bushes; the second one, is of intracellular thick hyphae that form circumvolutions.
The presence of hyphae Paris and Arum in arbuscular mycorrhizal colonization, is determined by the interaction between the genotype of the plant and the genotype of the mycorrhizal fungus of the host plant, however, the establishment of mycorrhizal association in natural conditions can be considered as multicausal (Santillana and Toro, 2018). Other authors mention that, following the proposed classification of AMF colonization types, corresponds to the type called Intermedite I, which is characterized by the presence of intracellular shrubs formed from intercellular hyphae and intracellular hyphae informing for the first time the presence of this relationship in the temperate forests of the southern hemisphere, thus it is valued that the hyphae found in this research, are characteristics corresponding to a mycorrhizal shrub (Cottet and Messuti, 2017).
The density of spores found after screening the soils is shown in Figure 4, which shows that the lowest amount of spores was obtained in the saplings category in the Guayaquil plot (30.8 %) and the maximum value was obtained in this same sampling site in the Fustal category (57.6 %), finding a statistically significant difference when comparing the results between the three categories analyzed, precisely between Saplings and Fustal.
The values obtained differ quantitatively from the results reported by Peña and López (2018), who when evaluating the presence of spores of arbuscular mycorrhizal fungi in five successional states of the Colombian high Andean forest detected between 4 and 20 g-1 spores; however, the highest values in the number of spores were found in the mature successional state, which corroborates the maximum value obtained in the fustal category of this research.
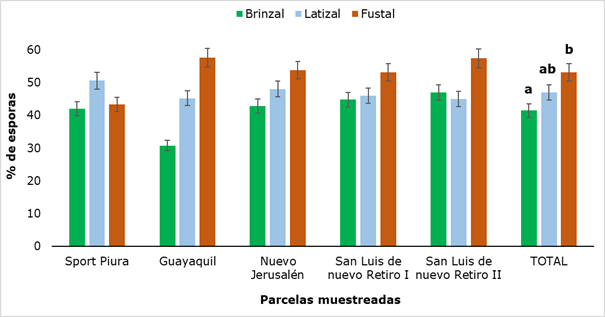 Different letters show that there were significant differences (P ˂ 0.05) between the developmental stages analyzed.
Different letters show that there were significant differences (P ˂ 0.05) between the developmental stages analyzed.Fig. 4. - Percentage of spores in rhizospheric soil of R. rospigliosii
Mycorrhizal spores in the soil are the largest (between 145 and 800 µm), with colors ranging from hyaline and pale yellow to brown, reddish and black. The walls of the spores (smooth or ornamented), the size and the ontogeny of the spores, form a basis for the identification of these fungi. These characteristics are very useful for the taxonomic identification of AMF, which allowed in this research to identify three AMF genera: Glomus, Entrophosora and Acaulospora, as shown in Figure 5.
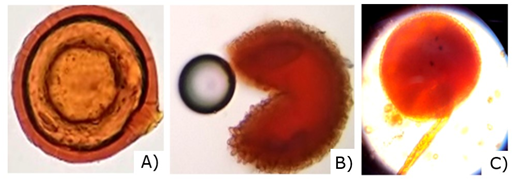
Fig. 5. - Spores found in the rhizosphere of R. rospigliosii Pilger. A) Glomus sp., B) Entrophosora sp., C) Acaulospora sp.
When evaluating the presence of vesicles in the roots (Figure 6) it was found that this structure was present in all sampled plots and values ranged from 35 % (Fustal category in Guayaquil) to 51.4 % (saplings category in Sport Piura).
In general, the highest values were found in the saplings category, which showed significant difference with the latizal category. Similar results were found by Garcia et al. (2004) in a research with eight forest species in the high Andean forest, reporting maximum values of 51.7% of vesicles.
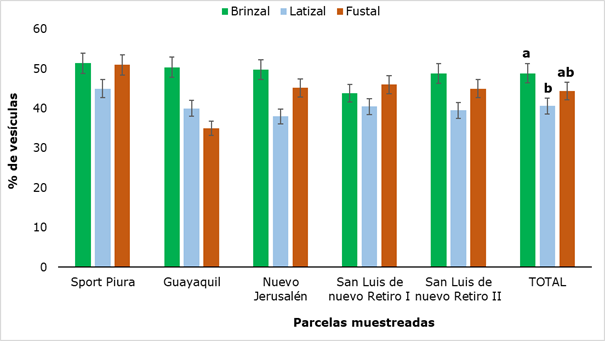 Different letters show that there were significant differences (P ˂ 0.05) between the developmental stages analyzed.
Different letters show that there were significant differences (P ˂ 0.05) between the developmental stages analyzed.Fig. 6. - Percentage of vesicles present in rhizospheric soil of R. rospigliosii
These portions of hyphae (vesicles), which form some genera of AMF, are present intercellularly in the root bark and are considered nutrient reservoirs for the fungus (Figure 7). This symbiosis between the AMF and the plant is known as vesicle-arbuscular (VA) due to the presence of vesicles and shrubs; since not all species of fungi develop vesicles, they are currently known as arbuscular mycorrhiza (Camarena-Gutiérrez, 2012).
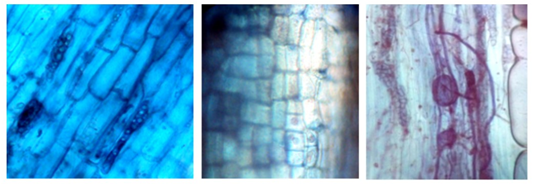
Fig. 7. - Vesicles in the category saplings colored with Trypane blue in R. rospigliosii, observed at 40X
The process of infection or colonization occurs initially with the growth of the fungus in the soil and physical contact with the root. Then, an exchange of information occurs that allows the fungus to grow between the root cells and colonize the plant tissue. In some places, the hypha enters the cell wall and divides intensely, but does not break the plasma membrane. This branched structure is called a bush and is recognized as an organ of metabolism exchange between partners (Ortiz-Acevedo et al., 2015).
The behavior of the presence of shrubs, in the evaluations exposed in the present investigation, was different from the other analyzed structures (hyphae, spores and vesicles), because it was not detected in all the plots, and in some it was not registered in the three evaluated categories (Figure 8) .
As can be seen in Figure 8, in San Luis de Nuevo Retiro II no shrubs were found and in San Luis de Nuevo Retiro I they were detected, in all three categories evaluated. In the rest of the sampled plots there is a presence of shrubs, but not in all categories, hence there are statistically significant differences between the results obtained for each sampling site. The values ranged from a minimum of 0.6 % to a maximum of 2.0 %, the former in the latizal and fustal categories and the latter in the saplings category. In Figure 9, some shrubs found in the latter category are shown.
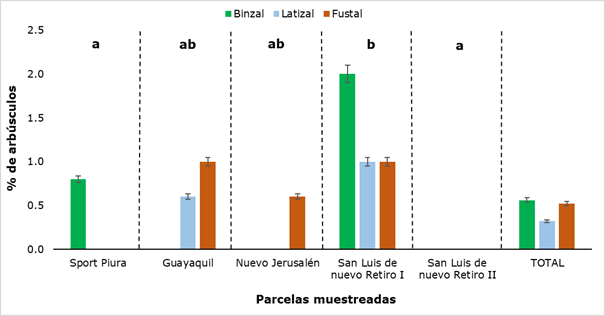 Different letters show significant differences (P ˂ 0.05) between the sampled plots.
Different letters show significant differences (P ˂ 0.05) between the sampled plots.Fig. 8. - Percentage of bushes detected in the different sectors and categories of R. rospigliosii
The results show a low percentage of shrubs, when compared with other researches, such as those carried out by Parra-Rivero et al., (2018) and Rivera et al., (2016), who report high values for this structure (between 31 % and 90 %), when studying the colonization of AMF in tree plants. This difference in the presence of shrub-type colonizing structures may be due to the fact that these have an ephemeral life span (4-15 days) and always end up being digested by the host plant (Rivera et al., 2016; Pérez et al., 2016).
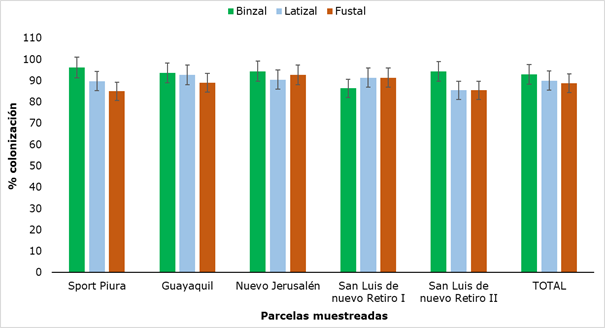
The results of AMF colonization in Retrophyllum rospigliosii Pilger are shown in Figure 10, where the minimum value obtained was 85 % (fustal) and the maximum was 96.2 % (saplings).
The values obtained for colonization did not show significant differences when analyzing the sampled plots or when comparing the three categories. Although these values do not have a standardized pattern of comparison, since the variability of mycorrhizal colonization is associated with the type of plant species and its physiological age (Belezacaet al., 2020), they do constitute very high values of colonization, which together with the presence of mycorrhizal structures in all categories, allows us to affirm that there is a certain degree of dependence between the plant and the AMF, an element of great importance because, in the mycorrhizal symbiosis, the fungus allows the increase in the acquisition of nutrients, mainly those of scarce mobility in the soil, such as phosphorus, at the same time that the plant provides carbonate compounds for the growth of the fungus (Flores and Cuenca, 2004).
This element becomes more important in this research because in the Humantanga forest, as reported by UNC (2019), phosphorus is at a low level (8.11 ppm), taking into account the interpretative ranges mentioned by Andrades and Martinez (2014) . In this case, the fungal activity associated to the plant generates a system of mutual benefit, because the AMFs modify the morphology of the roots, giving the plant a better capacity to reach the water and dissolve the nutrients, being able to solve the problem of the imminent exhaustion of the phosphate stock and other elements like, nitrogen, zinc, copper, iron, potassium, calcium and magnesium (in some cases the nutrients may control development or initiate symbiosis), may also cause a change in absorption of more nutrients at the same time and allow induction of host defenses to enhance nutrition (Uc et al., 2019; Piliarová et al., 2019).
Conclusions
In all the growth categories of Retrophyllum rospigliosii Pilger stands analyzed in the research, typical structures of AMF (hyphae, spores, vesicles and shrubs) were found, indicating that this is a plant species that symbiosizes with arbuscular mycorrhizae.
The genera of fungi determined in the three categories saplings, latizal and fustal of R. rospigliosii Pilger, according to the morphology of the spores, correspond to Glomus, Entrophospora and Acaulospora.
The high percentages of AMF colonization in R. rospigliosii Pilger and its presence in the three development categories, allow us to affirm that this symbiotic relationship is important in the development of male rosemary in the Huamantanga forest, guaranteeing a good absorption of phosphorus, in addition to the other benefits derived from this fungus-plant interaction.
These results contribute to the knowledge of this mycorrhizal colonization, which allows to value the use of AMFs in the development of cultural techniques in reforestation programs of R. rospigliosii Pilger, a forest species declared as vulnerable.
Referencias bibliográficas
ACUÑA, N.R. y MARCHANT, C., 2016. ¿Contribuyen las prácticas agroecológicas a la sustentabilidad de la agricultura familiar de montaña? El caso de Curarrehue, región de la Araucanía, Chile. Cuadernos de Desarrollo Rural, vol. 13. Bogotá: Pontificia Universidad Javeriana. Núm. 78, julio-diciembre, pp. 35-66. ISSN: 0122-1450. Disponible en: http://www.redalyc.org/articulo.oa?id=11753256002 [ Links ]
ANDRADES, M. y MARTÍNEZ, M.E., 2014. Fertilidad del suelo y parámetros que la definen. 3ra ed. Logroño: Universidad de la Rioja, Servicio de Publicaciones, 29p. ISBN 978-84-695-9286-1. Disponible en: https://dialnet.unirioja.es/servlet/libro?codigo=267902 [ Links ]
ARMENTERAS, D., GONZÁLEZ, T.M., VERGARA, L.K., LUQUE, F.J., RODRÍGUEZ, N., BONILLA, M.A., 2015. Revisión del concepto de ecosistema como "unidad de la naturaleza" 80 años después de su formulación. Ecosistemas, vol 25 no. 1, pp. 83-89. Disponible en: https://doi.org/10.7818/ECOS.2016.25-1.12 [ Links ]
BAÑUELOS, J., SANGABRIEL, W., GAVITO, M.E., TREJO, D., CAMARA, S., MEDEL, R., y CARREON, J., 2017. Efecto de diferentes niveles de fósforo en aguacate inoculado con hongos micorrízicos arbusculares. Revista Mexicana de Ciencias Agrícolas, Vol. 8, Núm. 7, pp: 1509-1520. Disponible en: http://www.scielo.org.mx/pdf/remexca/v8n7/2007-0934-remexca-8-07 -1509.pdf [ Links ]
BELEZACA, C., CALLE, D., PRIETO, O., LÓPEZ, R., SOLANO, E., DÍAZ, O., DÍAZ, P., GUACHAMBALA, M., BOHÓRQUEZ, T., 2020. Hongos de micorriza arbuscular presentes en Ochroma pyramidale (Cav. ex Lam.) Urb. (Balsa) en Ecuador. Journal of Science and Research, Vol. 5, Nº 3, pp: 1-14. E-ISSN: 2528-8083. Disponible en: https://revistas.utb.edu.ec/index.php/sr/article/view/899 [ Links ]
BONILLA, J.A. y ALARCÓN, J.A., 2015. Turnos técnico y económico de tala para árboles de Romerillo blanco en Ecuador. Ecología Aplicada, vol. 14 No. 2, pp. 127-137. Disponible en: http://www.scielo.org.pe/scielo.php?script=sci_arttext&pid=S1726-22162015000200005 [ Links ]
CAMARENA-GUTIÉRREZ, G., 2012. Interacción planta-hongos micorrízicos arbusculares.. Revista Chapingo Serie Ciencias Forestales y del Ambiente, Vol. XVIII, Núm. 3, pp. 409-421. DOI: Disponible en: http://dx.doi.org/10.5154/r.rchscfa.2011.11.093 [ Links ]
COTTET, A.C. y MESSUTI, M.I., 2017. Identificación del tipo morfológico de micorriza arbuscular en Phaeoceros laevis (Anthocerotophyta). Bol. Soc. Argent. Bot, vol. 52 no.2. pp. 291-293. Disponible en: https://botanicaargentina.org.ar/wp-content/uploads/2017/06/08_cottet.pdf [ Links ]
FAO, 2016. El estado de los bosques del mundo. Los bosques y la agricultura: desafíos y oportunidades en relación con el uso de la tierra. Organización de las Naciones Unidas para la Alimentación y la Agricultura, Roma, 137p. ISBN 978-92-5-309208-6. Disponible en: http://www.fao.org/3/a-i5588s.pdf [ Links ]
FLORES, C. y CUENCA, G., 2004. Crecimiento y dependencia micorrízica de la especie pionera y polenectarífera Oyedaea verbesinoides (Tara amarilla), Asteraceae. Interciencia, vol. 29, no. 11, pp. 632-637. Disponible en: http://ve.scielo.org/scielo.php?script=sci_arttext&pid=S0378-18442004001100007 [ Links ]
GARCÍA, J.F., GARCÍA, D.C. y CORREA, M., 2004. Incidencia de las micorrizas arbusculares y vesículo arbusculares como estrategia adaptativa de especies de páramo y selva altoandina, cordillera oriental de Colombia. Colombia Forestal, vol. 8, no. 17, pp: 43-59. Disponible en: https://doi.org/10.14483/udistrital.jour.colomb.for.2004.1.a03 [ Links ]
GARZÓN, L.P., 2016. Importancia de las Micorrizas Arbusculares (Ma) para un uso sostenible del suelo en la amazonia colombiana. Luna Azul, no. 42, pp: 217-234. Disponible en: https://doi.org/10.17151/luaz.2016.42.14 [ Links ]
GUERRERO, E. y HODSON, E., 1988. Micorrizas en Decussocarpus rospigliossi (Pilger) de Laubenfels, una Podocarpacea del bosque andino. Universitas Scientiarum, vol. 1 no. 2, pp. 53-66. Disponible en: http://revistas.javeriana.edu.co/index.php/scientarium/article/view/5091 [ Links ]
LATTUADA, D.S., RIETH, S., BACK, M.M. y DE SOUZA, P.V.D., 2019. Interaction between endomycorrhizae and native fruit tree (myrtaceae) in Rio Grande do Sul state. Ciencia Florestal, vol. 29 no. 4, pp: 1726-1736. Disponible en: https://doi.org/10.5902/1980509837389 [ Links ]
MEDINA-GARCÍA, L.R., 2016. La agricultura, la salinidad y los hongos micorrízicos arbusculares‰§ una necesidad, un problema y una alternativa. Revista Cultivos Tropicales, vol. 37, Núm. 3, pp. 42-49. Disponible en: http://dx.doi.org/10.13140/RG.2.1.1117.9765 [ Links ]
OCHOA, R., 2017. Consecuencias de la deposición de nitrógeno sobre la biodiversidad y el funcionamiento de los ecosistemas terrestres: una aproximación general desde la ecología de los sistemas. Revista Ecosistemas, vol. 26 no. 1, pp. 25-36. ISSN 1697-2473. Disponible en: http://doi.org/10.7818/ecos.2017.26-1.05 [ Links ]
ORTIZ-ACEVEDO, A., OSORIO-VEGA, N.W., ECHEVERRI-GÓMEZ, J., GONZÁLEZ-MURILLO, O.A., y MEDINA-SIERRA, M., 2015. Fisiología de los hongos formadores de micorrizas arbusculares. Livestock Research for Rural Development, vol. 27, no. 9, pp. 188. Disponible en: http://www.lrrd.org/lrrd27/9/orti27188.html [ Links ]
PARRA-RIVERO, S.M., MACIEL-DE SOUSA, N.M., SANABRIA-CHOPITE, M. y PINEDA, J., 2018. Descripción anatómica de la colonización de hongos micorrízicos arbusculares en dos leguminosas arbóreas. Revista Chapingo Serie Ciencias Forestales y del Ambiente, vol. 24, no. 2, pp. 183-196. DOI: Disponible en: http://dx.doi.org/10.5154/r.rchscfa.2017.02.014 [ Links ]
PEÑA, A., ROJAS, M., MILÁN, E., GUERRERO, N. y ARIAS, H., 2019. Implementación de las técnicas agroecológicas de microorganismos eficientes y bioplaguicida en finca de productores. Talle Uso y manejos sostenibles de ecosistemas agrícolas, 9na Conferencia Científica Internacional de la Universidad de Holguín, Cuba. Disponible en: https://eventos.uho.edu.cu/index.php/ccm/ccm9/paper/view/4092 [ Links ]
PEÑA, D.Y. y LÓPEZ, F., 2018. Presencia de esporas de hongos micorrízico arbusculares en cinco estados sucesionales de bosque altoandino colombiano. Revista de Investigación Agraria y Ambiental, vol. 9, no. 2, pp. 135-148. DOI: Disponible en: https://doi.org/10.22490/21456453.2194 [ Links ]
PÉREZ, A., CURY, K. y OVIEDO, L., 2016. Colonización de micorrizas arbusculares en tres especies de pasturas del departamento de Sucre.Temas Agrarios, vol. 21 no. 2, pp. 65-75. Disponible en:https://doi.org/10.21897/rta.v21i2.902 [ Links ]
PÉREZ, Y. DEL C., ÁLVAREZ, P. E., GONZÁLEZ, D. y MÉNDEZ, V., 2019. Evaluación de la presencia de hongos micorrízico arbusculares en un bosque de pino-encino en Chiapas, México. Idesia, vol. 37, no. 1, pp. 67-73. Disponible en: http://dx.doi.org/10.4067/S0718-34292019005000401 [ Links ]
PHILIPS, J.M. y HAYMAN, D.S., 1970. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, Vol. 55, No.1, pp. 158-161. Disponible en: https://doi.org/10.1016/S0007-1536(70)80110-3 [ Links ]
PILIAROVÁ, M., ONDREIÈKOVÁ, K., HUDCOVICOVÁ, M., MIHÁLIK, D., KRAIC, J., 2019. Arbuscular mycorrhizal fungi their life and function in ecosystem. Agriculture (Polnohospodárstvo), Research Institute of Plant Production, Slovakia, vol. 65, no. 1, pp. 315. Disponible en: http://doi.org/10.2478/agri-2019-0001 [ Links ]
RIVERA, F.A., GONZÁLEZ, V., GONZÁLEZ, J.G. y OSSA, P.A., 2016. Caracterización molecular, análisis morfológico y colonización micorrízica en la rizósfera del aguacate (Persea americana Mill) en Caldas, Colombia. Revista Acta Agronómica, vol. 65, no. 4, pp. 398-405. DOI: Disponible en: https://doi.org/10.15446/acag.v65n4.51714 [ Links ]
SANTILLANA, N. y TORO, M., 2018. Asociación micorrízica arbuscular en pastizales de la comunidad alto andina de Ccarhuaccpampa Ayacucho. RevistaEcología Aplicada, vol. 17, Núm. 2, pp. 165-169. Disponible en: http://dx.doi.org/10.21704/rea.v17i2.1236 [ Links ]
SIEVERDING, E., 1983. Manual de métodos para la investigación de la micorriza vesículo-arbuscular en el laboratorio. Centro Internacional de Agricultura Tropical, Cali, Colombia. Disponible en: https://es.scribd.com/document/270011075/Manual-de-Metodos-Para-La-Investigacion-de-La-Micorriza-Vesiculo-Arbuscular-en-El-Laboratorio [ Links ]
UC, A.G., ARREOLA, J., CARRILLO, E., OSNAYA, M.M., ALARCÓN, A., FERRERA, R., y LANDEROS, C., 2019. Inoculación de hongos micorrízicos arbusculares en el cultivo de Heliconia stricta. Revista Mexicana de Ciencias Agrícolas, vol. 10 no. 5, pp. 1057-1069. Disponible en: https://doi.org/10.29312/remexca.v10i5.1608 [ Links ]
UNC, 2019. Proyecto Conservación del Romerillo macho Retrophyllum rospigliossi en bosques y plantaciones de Jaén, Perú. Informe final de resultados presentados a la Unidad de Investigación, Universidad Nacional de Cajamarca. [ Links ]
Received: August 11, 2020; Accepted: October 24, 2020











 texto en
texto en