My SciELO
Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Cubana de Ciencias Forestales
On-line version ISSN 2310-3469
Rev cubana ciencias forestales vol.9 no.1 Pinar del Río Jan.-Apr. 2021 Epub Apr 02, 2021
Original article
Phenolic composition of Cryptocarya alba (Mol) Looser in different locations and seasons of the year
1Universidad de Chile. Facultad de Ciencias Forestales y de la Conservación de la Naturaleza. Departamento de Silvicultura. Chile.
2Universidad de Chile. Facultad de Ciencias Agronómicas. Departamento de Agroindustria y Enologí a. Chile.
4Universidad de Chile. Facultad de Ciencias Forestales y de la Conservación de la Naturaleza. Departamento de Silvicultura. Chile
5Universidad de Chile. Facultad de Ciencias Forestales y de la Conservación de la Naturaleza. Chile.
The phenolic composition of various plant species has been studied in order to know quantitatively and qualitatively the compounds present in them, to be used as raw material in the food, pharmacological and cosmetic industries, among others. In Chile there is little information on the phenolic composition of native plant species of the Mediterranean forest, so it is of great importance to carry out phenolic characterization studies, in order to detect which are the major compounds for their possible extraction or use of raw materials. The objective of this study was to characterize and quantify by high performance liquid chromatography (HPLC-DAD) the phenolic composition of Cryptocarya alba (Mol) Looser, in young leaves and branches, in seven locations in central Chile and during the winter, spring and summer seasons. Total phenols (FT), condensed tannins (TC) and low molecular weight phenols were quantified in the different samples. In C. alba leaves, for the different seasons of the year and localities analyzed, concentration ranges of 9.83-29.85 mgEAG g-1 for FT and 7.06-32.00 mgEC g-1 for TC; while in the young branches, concentrations between 0.68-1.61 mgEAG g-1 for TF and 1.23-2.53 mgEC g-1 for TC. The low molecular weight phenolic compounds identified in the highest concentration in C. alba were: trans-chlorogenic acid, followed by procyanidins and flavonols. Finally, it was concluded that the composition and concentration of phenolic compounds of C. alba changes with the locality and season of the year.
Keywords: Phenolic compounds; Cryptocarya alba; Flavonoids; Tannins.
INTRODUCTION
In Chile there is little information on the phenolic composition of native plant species in the Mediterranean forest. Among the most important tree species in the Mediterranean region, Cryptocarya alba (Mol.) Looser (peumo), a tree endemic to Chile, stands out. It grows from the south of Limarí province (30° 30' S and 71° 00' W) to Cautín province (between 37° 35' and 39° 35' S) (Rodríguez et al., 1983). A potential use in traditional medicine, has this species, because for the genus Cryptocarya, have been described, approximately 40 alkaloids with antitumor properties, bactericides, antimicrobials, fungicides, insecticides or antioxidants (Avello et al., 2012; Di Cosmo et al., 2015; Bravo et al., 2017; Viktorová et al., 2020). Specifically, for C. alba, an alkaloid with hepatoprotective properties has been found isolated from leaves and bark (Vogel et al., 2008; Castro-Saavedra et al., 2016). Essential oils have also been isolated from its leaves, including p-cymol, alpha-pinene, linalol, limonene, borneol, beta-pinene, 1-terpinen-4-ol, betaterpinene, and eucalyptol (Vogel et al., 2008; Avello et al., 2012; Bravo et al., 2017), and flavonoids in leaves and stems, glycosides, and chlorogenic acid (Vogel et al., 2008; Castro-Saavedra et al., 2016). The variations that C. alba presents during the seasons are unknown, and it is also not known if there are changes in the concentrations of phenolic compounds depending on the location of the species.
The objective of this study was to characterize and quantify the phenolic composition of Cryptocarya alba, in leaves and young branches, in different locations and seasons. Knowing this answer will allow evaluating the potential of Cryptocarya alba as a source of phenolic compounds, and generating proposals of areas and seasons of better use of biomass extraction.
MATERIALS AND METHODS
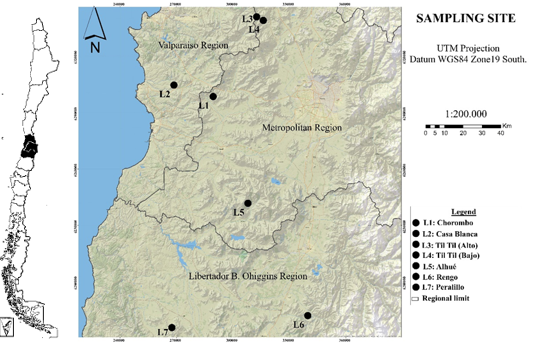
The sampling was carried out in seven locations in central Chile, during the winter (July 2009), spring (October 2009) and summer (December 2009-January 2010) seasons (Figure 1). The climatic conditions of the summer and winter period of these locations are shown in Table 1. In these locations, adult trees were selected, similar in size and development.
Table 1 - Average values of average temperatures and normal annual precipitation in the studied localities, for the period 1980-2010 (Santibáñez et al., 2016)
| Locality | Average temperature in summer (°C) | Average temperature in winter (°C) | Annual rainfall (mm) |
| Casablanca | 17,7 | 10,4 | 466 |
| Chorombo* | 19,3 | 9,6 | 472 |
| Til Til (alto) | 19,5 | 8,8 | 408 |
| Til Til (bajo) | 20,5 | 9,4 | 338 |
| Alhué | 19,4 | 8,6 | 554 |
| Rengo | 16,7 | 6,0 | 760 |
| Peralillo | 19,9 | 9,4 | 616 |
* The nearest weather station was used for this study: María Pinto.
Twigs 20 cm long, with a maximum diameter of 1.5 cm, were collected. The collection was made in the upper middle section of the adult tree crown, in four exhibitions of the same (North, South, East, West). These contained leaves from the current and previous year, fully developed, alive and functional. Leaves with symptoms of senescence were discarded. For the study of phenolic compounds (low molecular weight polyphenols), it was used a high-performance liquid chromatography equipment (HPLC-DAD) brand Agilent, model 1 200. A Nova-PaK (Waters) C-18 column (3.9 x 300 mm) was used. The separation was performed with a gradient with 2 % acetic acid (A), acetonitrile: acetic acid: water (20:2:78 %; B) and methanol (C). The elution program started with 100% A (1 ml s-1 flow) for 55 min; then, for 2 min, 20 % A and 80 % B (1.2 ml s-1 flow); for 33 min 10 % A and 90 % B (1.2 ml s-1 flow); finally, 100 % C for 2 min (1.2 ml s-1 flow). The detection wavelength was 280 nm, and the injection volume was 30 µl.
The standards used for the calibration curves were: caffeic, chlorogenic, ellagic, ferulic, gallic, protocatholic, vanillinic; (+)-catechin; (-)-epicatechin; quercetin; vanillinic aldehyde; trans-resveratrol, obtained from Sigma-Aldrich, 99.5% purity. The analyses were carried out with three repetitions, corresponding to three trees chosen in the sampling locations.
Sample preparation and extraction
The samples were processed by separating branches and leaves, which were dried at room temperature and stored in paper bags in a cool place in the dark. Until the moment of the analysis, the leaves had a moisture content between 12 and 15%. From each sample, 5 g of dried leaves and 10 g of dried twigs were ground separately. These were macerated in 100 ml of a solution of methanol: water 80:20 (v/v) for 2 hours. Afterwards, the samples were centrifuged at 3 500 rpm for 30 min. and then vacuum filtered using a 0.45 µm membrane. The extract obtained was used for all the analyses described below:
Analysis of phenolic compounds
The analysis of total phenols was obtained by measuring the optical density at 280 nm. The spectrophotometric readings were made in a UV-VIS PharmaSpec spectrophotometer, model UV-1700. The average results of the readings were expressed in mg gallic acid equivalents per gram of sample (mgEAG g-1) (Zoecklein et al., 2001).
The condensed tannins were measured by means of the Bate-Smith reaction, which consists in the transformation of proanthocyanidic tannins into anthocyanidins by heating at 95°C for 2 hours with a 5% solution of butanol in HCl (Bate-Smith, 1981). The results were reported as equivalent mg of catechin per g of extract (mgEC g-1).
For the extraction of the low molecular weight phenols, 50 ml of the extract obtained from each sample were taken, and then taken to a rotary evaporator at 35° C until 80 % of the volume was evaporated, in order to eliminate methanol. After completing the volume with distilled water up to 50 ml, it was subjected to three extractions with 20 ml ethyl acetate and then three extractions with 20 ml ethyl ether. To the organic extract obtained, an anhydrous sodium sulfate salt was added for 30 minutes and then filtered on paper, concentrating to dryness in a rotary evaporator at 35° C (Peña-Neira et al., 1999). The film obtained in the heart flask, once the sample was dried, was recovered with 2 ml of a solution of methanol: water (50:50; v/v), for its later analysis by HPLC-DAD, after filtration (0.45 µm).
Experimental design and statistical analysis
The design of the experiment was factorial, considering as factors the location and the season of the year. The experimental unit corresponded, in the case of the leaves, to 100, and in the case of the branches, to 5. Analysis of Variance (ANDEVA) was carried out for all the obtained variables. If there were significant differences, it was applied the Duncan's Multiple Range Test with a confidence level of 95 %.
RESULTS
Phenolic composition in leaves: total phenols and condensed tannins
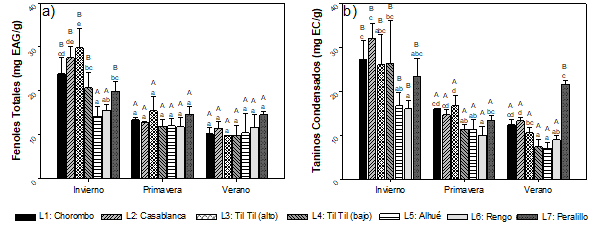
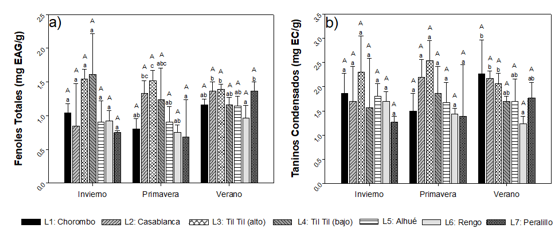
For total phenols (TF) (Figure 2)a a concentration variation of 9.83 ± 0.05 mgEAG g-1 to 29.85 ± 4.39 mgEAG g-1 was observed between the winter and spring seasons, with the highest contents occurring in the Til Til locality (high) in both seasons. TF concentrations were significantly higher in winter, increasing by 40 % on average. In winter, greater variability of TF was observed between the localities, while in spring and summer the TF content did not present significant differences between them.
Condensed tannins (TC) (Figure 2)b, in general, presented a concentration range between 7.06 ± 1.36 mgEC g-1 and 32.00 ± 3.46 mgEC g-1, showing the minimum value in the town of Alhué (summer) and the maximum, in Casablanca (winter). For CT, the highest concentrations were also presented in winter. The results showed that the geographical factor or location affects the content of phenolic compounds significantly, in the three seasons of the year.
Phenolic composition in leaves: low molecular weight phenols
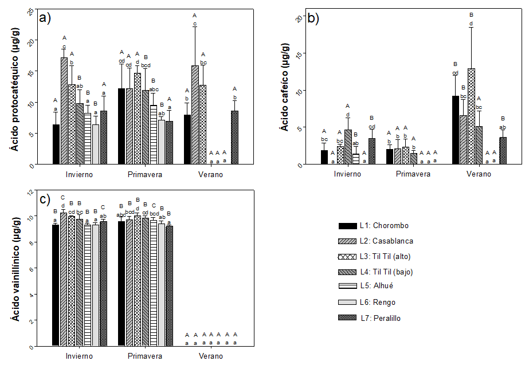
No flavonoids. All of these compounds increased their concentration during the spring, and in most of these they decreased their concentration or were not identified during the summer (Figure 3). The locations of Casablanca and Til Til (alto), on average, had the highest concentrations of this group of compounds. For cis-chlorogenic acid, concentrations ranging from 3.26 ±1.85 µg g-1 to 34.22 ± 6.61 µg g-1 were observed during winter and spring, not being identified in summer (Figure 3)a. For trans-chlorogenic acid (Figure 3) bconcentrations between 0.43 ± 0.12 mg g-1 and 13.21 ± 6.23 mg g-1 were detected, with the highest concentration in the locality of Casablanca, during the spring. For caffeic acid, the maximum concentration of 14.59 ± 4.71 µg g-1 was obtained in the Til Til (bajo) locality, during spring, and in this same locality, this compound could not be detected in summer. The results show that there is an interaction between the factors analyzed, with significant differences observed between seasons and between locations, with spring being the time of greatest concentration (Figure 3)c. Figure 3d shows the content of ferulic acid, with concentrations similar to those observed for caffeic acid. The highest average concentration was observed in spring compared to winter. There is also an interaction between the factors analyzed for the concentration of caffeic acid ester, showing significant differences between seasons and between localities. The differences between the localities are present in spring and summer (Figure 3)e. In the case of ferulic acid ester, concentrations between 3.25 ± 2.95 µg g-1 and 42.76 ± 13.92 µg g-1 were obtained between the stations and localities analyzed.
There is an interaction between the factors studied and significant differences between the stations (Figure 3)f. Protocathic acid (Figure 3)g, presents concentration ranges between 0 µg g-1 and 9.5 µg g-1 between the stations and locations analyzed, presenting significantly higher concentrations in spring.
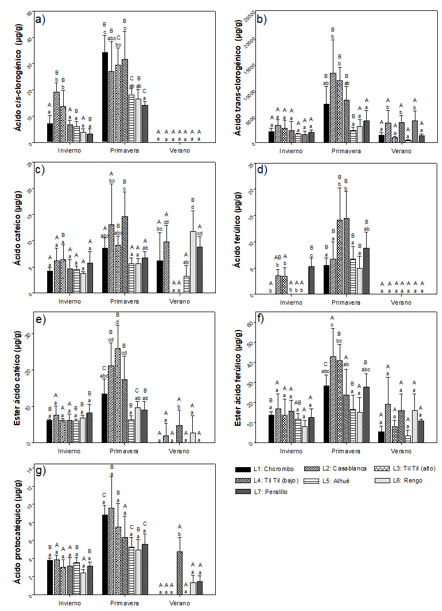
Capital letters indicate significant differences between seasons and small letters between locations analyzed (n=3).
Fig. 3 - Non-flavonoid phenolic compounds (cis-chlorogenic acid (a), trans-chlorogenic acid (b), caffeic acid (c), ferulic acid (d), caffeic acid ester (e), ferulic acid ester (f), protocatholic acid (g)) present in Cryptocarya alba leaves, according to location and season
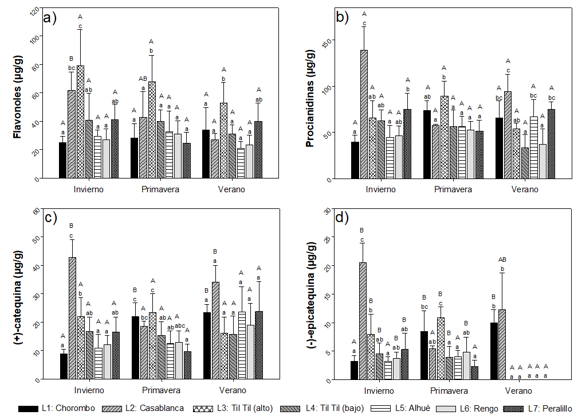
Flavonoids. The same trend was observed as the non-flavonoid compounds, showing higher concentrations in spring (Figure 4). Also, the localities of Casablanca and Til Til (alto) showed the highest concentrations.
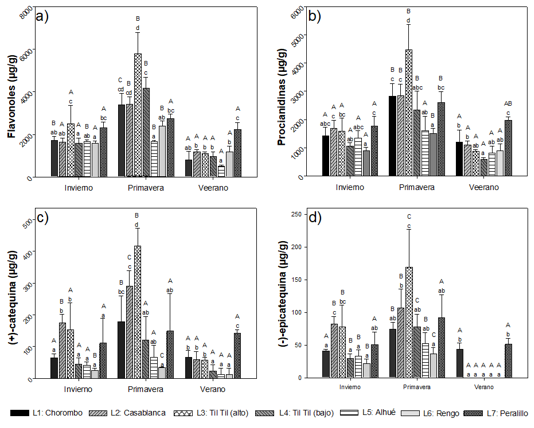
Capital letters indicate significant differences between seasons and small letters between locations analyzed (n=3).
Fig. 4 - Flavonoid phenolic compounds (flavonols (a), procyanidins (b), (+)-catechin (c), (-)-epicatechin (d)) present in leaves of Cryptocarya alba, according to location and season
For the flavonols (corresponding to the sum of all identified flavonols), average concentrations were observed between 0.49 ±0.05 mg g-1 and 5.7 ±0.99 mg g-1, where the minimum value corresponded to the town of Alhué in summer, while the maximum was recorded in the town of Til Til (alto) in spring. Significantly higher concentrations were found in the spring than in the other seasons (Figure 4)a.
Procyanidins have concentrations between 0.58 ± 0.06 mg g-1 and 4.47 ± 0.88 mg g-1. Interaction between season and location was observed, and significant differences between seasons, since in spring, significantly higher average concentrations were obtained (Figure 4)b. Concentrations between 13.3 ± 2.6 µg g-1 and 415 ± 56.34 µg g-1 were observed for (+)-catechin, with the minimum value corresponding to Rengo locality, in summer, and the maximum, for Til Til locality, in spring (Figure 4)c. Figure 4d shows concentrations between 0 µg g-1 and 169.92 ± 57.59 µg g-1 for (-)-epicatechin. There is a relationship between the seasons of the year, the locations and the content of (+)-catechin and (-)-epicatechin, observing significant differences between the seasons, being in spring where the concentrations are significantly higher for both compounds, when compared to the other seasons of the year (70 % for (+)-catechin and 84% for (-)-epicatechin. The summer season is the one with the lowest concentration. Among the localities, there are significant differences in the three seasons, for both compounds. In winter, it is observed that the highest average concentrations, are present in the locality Casablanca and Til Til (alto), and in spring, in the locality of Til Til (alto).
Phenolic composition in young branches: total phenols and condensed tannins
Concentrations between 0.68 ± 0.55 mgEAG g-1 and 1.61 ± 0.6 mgEAG g-1 for TF were observed in the young branches of C. alba; the minimum value corresponded to Peralillo, in spring and the maximum to Til Til (bajo), in winter (Figure 5)a. On the other hand, concentrations between 1.23 ± 0.15 mgEC g-1 and 2.53 ± 0.41 mgEC g-1 for CT were found. The minimum value corresponded to Rengo, in summer, and the maximum value to Til Til (alto), in spring (Figure 5)b. Significant differences were found between the localities, for both compounds, which occurred in spring and summer, for TF, and only in summer, for CT.
Upper case letters indicate significant differences between seasons and lower-case letters between locations analyzed (Mean concentrations ± standard deviation with n=3).
Fig. 5 - Total phenols (a) and condensed tannins (b) present in young branches of Cryptocarya alba, according to location and season
Phenolic composition in young branches: low molecular weight phenols
No Flavonoids. The non-flavonoid compounds identified in C. alba branches were three: protocathic acid, caffeic acid and vanillinic acid (Figure 6). Particularly no vanillinic acid was found in the summer period in any location. The concentration of caffeic acid tended to increase towards summer and only in Rengo was the compound not detected in any period.
Capital letters indicate significant differences between seasons and small letters between localities analyzed (n=3).
Fig. 6 - Non-flavonoid phenolic compounds (protocathic acid (a), caffeic acid (b), vanillinic acid (c)) present in young branches of Cryptocarya alba, according to location and season
Flavonoids. The flavonoid compounds found in young branches of C. alba are the same compounds that are present in the leaves (Figure 4 Figure 7), but the concentration is 90 % lower.
Capital letters indicate significant differences between seasons, and lowercase letters, between localities analyzed (n=3).
Fig. 7 - Flavonoid phenolic compounds (flavonols (a), procyanidins (b), (+)-catechin (c), (-)-epicatechin (d)) present in young branches of Cryptocarya alba, according to location and season
DISCUSSION
The composition and concentration of phenolic compounds present in C. alba leaves varied with location and season, as reported in other plant species. In boreal forests, Betula pubescens Ehrh leaves were observed present a wide difference in the accumulation of phenolic compounds depending on the season, finding that proanthocyanidin concentrations increase as the summer season progresses. However, procyanidins attached to the cell wall, gallotannins and glycosylated flavonoids, lower their concentration after an initial increase in young leaves (Wam et al., 2017). In the case of Quercus robur L. leaves, a seasonal variation of phenolic compounds was also found, in addition to total proanthocyanidins increasing throughout the summer season and other compounds such as total hydrolyzable tannins, glycosylated flavonoids, quercetin glycosides and kaempferol glycosides decreasing in concentration over the same period (Salminen et al., 2004; Burlacu et al., 2020). Geographic location is also a factor in the accumulation of phenolic compounds in plants, since climatic and nutritional conditions affect the activity of Phenylalanine ammonia (Kováèika et al., 2007; Sharma et al., 2019).
Although in recent years global climate change has modified both the temperatures and the amount of precipitation in the study area (showing a rate of increase of 0.13°C per decade and accentuating drought episodes), these changes have affected the entire central zone of Chile in a similar way (Villarroel et al., 2020). The confirmation of the results presented is reinforced by the work of Giordano et al., (2019), who also indicated that the locality of Til Til (Cuesta La Dormida) presented the highest contents of phenolic compounds in the aerial part of C. alba.
In the case of young C. alba branches, the concentration was lower than that shown on the leaves, but no significant differences were observed for TF and CT between seasons. Therefore, to continue with this type of study and determine the appropriate time of extraction of these compounds, it is better to use leaves. Species such as Vaccinium angustifolium Aiton and Vaccinium myrtillus L. also show a higher concentration of phenolic compounds in their leaves than in their branches, roots and fruits (Harris et al., 2007; Witzell et al., 2003; Stefãnescu et al., 2019).
It was observed a variation in the composition and concentration of non-flavonoid phenols in leaves and branches of C. alba, and between localities and seasons. Trans-chlorogenic acid was the majority compound identified in C. alba leaf extracts. The maximum concentration found of trans-chlorogenic acid (13 mg g-1) was low compared to other plant species, such as Ilex paraguariensis A.St.-Hil., with concentrations of 97 mg g-1(Marques and Farah 2009; Meinhart et al., 2019), and Coffea canephora Pierre ex A. Froehner, with concentrations of 95 mg g-1(Farah and Donangelo 2006; Pérez-Hernández et al., 2013).
In C. alba leaves, cis-chlorogenic acid and ferulic acid totally decreased their concentration in summer and in all localities. This suggests a behavior typical of C. alba, associated with reduced soil moisture, high solar radiation and high temperatures. For young branches it was also observed a behavior attributed to the species, so the concentration of vanillinic acid was totally reduced in summer, and in all localities. A similar behavior has been described for branches of Juglans regia L. with a decrease from spring to summer for non-flavonoid compounds such as chlorogenic and vanillinic acids (Solar et al., 2006; Binbin et al., 2017). The decrease towards the end of the growing season in the content of non-flavonoid phenolic compounds can result from three main causes: (1) decreasing rate of their biosynthesis, (2) conversion into insoluble components bound to the cell wall, and (3) active transformation into oligo- and polymeric compounds, e.g., tannins or lignins. In addition, these consequences may be due, in part, to a dilution effect by an increasing relative content of cell wall components, in the leaf and branches such as cellulose, hemicellulose, pectin and lignin (Nurmi et al., 1996; Vanholme et al., 2019).
One of the most documented mechanisms of adaptation to UV-B radiation is the increased production of secondary metabolites such as phenols and flavonoids, which accumulate in the cells of the epidermis of various plant species and because they are compounds that absorb radiation between 280-360 nm, they reduce the deleterious effect of UV-B light on the various cellular components (Rozema et al., 2002; Klein et al., 2018). Flavonoids perform functions associated with the plant's response to light and control the levels of auxins that regulate plant growth and differentiation (Martínez-Florez et al., 2002; Saxena et al., 2012).
In branches of some walnut cultivars Solar et al., (2006) and Binbin et al., (2017) observed for flavanols such as (+)-catechin and flavonols such as myricetin, a higher concentration in spring than in the other seasons of the year, which is consistent with the differences between seasons for these groups of compounds in C. alba.
CONCLUSIONS
The composition and concentration of phenolic compounds vary with location and season, in leaves and young branches of Cryptocarya alba. The leaves have higher concentrations of phenolic compounds than those found in the branches. Specifically, the maximum concentrations of phenols and total tannins, in leaves, are observed in the winter season. In the case of the branches, the concentration remains constant. The majority phenolic compound identified in this species was trans-chlorogenic acid, followed by procyanidins and flavonols. Regarding the low molecular weight phenolic compounds in Cryptocarya alba leaves, they increase their concentration in the spring season, and most of them decrease their concentration, or are not identified, during the summer. Therefore, it is necessary to identify the possible uses of the leaves with the time of harvest. The best time to extract phenolic compounds from Cryptocarya alba leaves would be the winter-spring season. The best sectors were Til Til (alto) and Casablanca. The higher concentrations of total phenols and condensed tannins found in Cryptocarya alba leaves allow us to assume a good potential for use in the food, pharmaceutical and cosmetic industries.
Acknowledgements
To Forest Engineer Marie Claire Aravena for her support in the elaboration of the image. This study has been carried out thanks to the project "Proposals for management models and conservation of biodiversity in Mediterranean forests. Domeyko Biodiversity Program, Vice-Rectory of Research and Development, University of Chile.
REFERENCIAS BIBLIOGRÁFICAS
AVELLO LORCA, M., LÓPEZ CANALES, C., GATICA VALENZUELA, C., BUSTOS CONCHA, E., BRIEVA CHAIT, A., PASTENE NAVARRETE, E. y BITTNER BERNER, M., 2012. Efectos antimicrobianos de extractos de plantas chilenas de las familias Lauraceae y Atherospermataceae. Revista Cubana de Plantas Medicinales [en línea], vol. 17, no. 1, pp. 73-83. [Consulta: 21/09/2020]. ISSN 1028-4796. Disponible en: Disponible en: http://scielo.sld.cu/scielo.php?script=sci_abstract&pid=S1028-47962012000100008&lng=es&nrm=iso&tlng=es . [ Links ]
BATE-SMITH, E.C., 1981. Astringent tannins of the leaves of Geranium species. Phytochemistry [en línea], vol. 20, pp. 211-216. Disponible en: https://www.semanticscholar.org/paper/Astringent-tannins-of-the-leaves-of-Geranium-Bate-smith/9d83ba2e735c197194f6ac07cca90ea79c4938fd . [ Links ]
BINBIN, S., WENE, Z., XUE, L. & XUEJUN, P., 2017. Seasonal variations of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks, International Journal of Food Properties, 20:sup3, S2635-S2646. Disponible en: https://www.tandfonline.com/doi/full/10.1080/10942912.2017.1381706 [ Links ]
BRAVO, J., CARBONELL, V., SEPÚLVEDA, B., DELPORTE, C., VALDOVINOS, C.E., MARTÍNHERNÁNDEZ, R., HIGES, M., 2017. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae, Journal of Invertebrate Pathology. vol. 149, pp 141-147. Disponible en: https://www.sciencedirect.com/science/article/abs /pii/S0022201117301532?via%3Dihub [ Links ]
BURLACU, E., NISCA, A., y TANASE, C., 2020. A Comprehensive Review of Phytochemistry and Biological Activities of Quercus Species. Forests. Vol 11 no. 9, 904-928. Disponible en: https://www.mdpi.com/1999-4907/11/9/904 [ Links ]
CASTRO-SAAVEDRA, S.; FUENTES-BARROS, G.; TIRAPEGUI, C.; ACEVEDO-FUENTES, W.; CASSELS, B.K.; BARRIGA, A.; VILCHES-HERRERA, M. 2016.Phytochemical analysis of alkaloids from the chilean endemic tree Cryptocarya alba. Journal of the Chilean Chemical Society, 61, 3076-3080. Disponible en: https://scielo.conicyt.cl/scielo.php?script=sci_arttext&pid=S0717-97072016000300014 . [ Links ]
DI COSMO, D., SANTANDER, R., URZÚA, A., PALACIOS, S., ROSSI, Y. 2015. Insecticidal effect of Cryptocarya alba essential oil on the housefly, Musca domestica L. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas vol 14 no. 2 113 117. Disponible en: https://www.redalyc.org/pdf/856/85636183006.pdf [ Links ]
FARAH, A. y DONANGELO, C.M., 2006. Phenolic compounds in coffee. Brazilian Journal of Plant Physiology [en línea], vol. 18, no. 1, pp. 23-36. [Consulta: 21/09/2020]. ISSN 1677-0420. DOI 10.1590/S1677-04202006000100003. Disponible en: Disponible en: http://www.scielo.br/scielo.php?script=sci_abstract&pid=S1677-04202006000100003&lng=en&nrm=iso&tlng=en . [ Links ]
GIORDANO A., FUENTES-BARROS, G., CASTRO-SAAVEDRA, S., GONZÁLEZ-COOPER, A., SUÁREZ-ROZAS, C., SALAS-NORAMBUENA J., ACEVEDO-FUENTES, W., LEYTON, F., TIRAPEGUI, C., ECHEVERRÍA, J., CLARO, S., CASSELS, B. 2019. Variation of Secondary Metabolites in the Aerial Biomass of Cryptocarya alba. Natural Product Communications. pp 1-11. Disponible en: https://journals.sagepub.com/doi/10.1177/1934578X19856258?icid=int.sj-abstract.similar-articles.2 [ Links ]
HARRIS, C.S., BURT, A.J., SALEEM, A., LE, P.M., MARTINEAU, L.C., HADDAD, P.S., BENNETT, S.A.L. y ARNASON, J.T., 2007. A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochemical Analysis [en línea], vol. 18, no. 2, pp. 161-169. [Consulta: 21/09/2020]. ISSN 1099-1565. DOI 10.1002/pca.970. Disponible en: Disponible en: https://onlinelibrary.wiley.com/doi/abs/10.1002/pca.970 . [ Links ]
KLEIN, FÁTIMA ROSANE S., REIS, ANDRESSA, KLEINOWSKI, ALÍTCIA M., TELLES, RENATA T., AMARANTE, LUCIANO DO, PETERS, JOSÉ A., & BRAGA, EUGENIA JACIRA B., 2018. UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Botanica Brasilica, vol 32 no. 4, 615-623. Disponible en: https://www.scielo.br/scielo.php?script=sci_arttext&pid=S0102-33062018000400615 [ Links ]
KOVÁÈIK, J., KLEJDUS, B., BAÈKOR, M. y REPÈÁK, M., 2007. Phenylalanine ammonia-lyase activity and phenolic compounds accumulation in nitrogen-deficient Matricaria chamomilla leaf rosettes. Plant Science [en línea], vol. 172, no. 2, pp. 393-399. [Consulta: 21/09/2020]. ISSN 0168-9452. DOI 10.1016/j.plantsci.2006.10.001. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S016894520600272X . [ Links ]
MARQUES, V. y FARAH, A., 2009. Chlorogenic acids and related compounds in medicinal plants and infusions. Food Chemistry [en línea], vol. 113, no. 4, pp. 1370-1376. [Consulta: 21/09/2020]. ISSN 0308-8146. DOI 10.1016/j.foodchem.2008.08.086. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S0308814608010649 . [ Links ]
MARTÍNEZ-FLOREZ, S., GONZÁLEZ, J., CULEBRAS, J. y TUÑÓN, M., 2002. Los flavonoides: propiedades y acciones antioxidantes. Nutr. Hosp, vol. 17, no. 6, pp. 1.370-1.376. Disponible en: http://www.nutricionhospitalaria.com/pdf/3338.pdf [ Links ]
MEINHART, A.D., et al., 2017. Chlorogenic acid isomer contents in 100 plants commercialized in Brazil. Food Research International. Vol 99 no. 1: 522-530. Disponible en: https://www.sciencedirect.com/science/article/abs/pii/S0963996917302697?via%3Dihub [ Links ]
NURMI, K., OSSIPOV, V., HAUKIOJA, E. y PIHLAJA, K., 1996. Variation of total phenolic content and individual low-molecular-weight phenolics in foliage of mountain birch trees (Betula pubescens ssp.tortuosa). Journal of Chemical Ecology, vol. 22, no. 11, pp. 2023-2040. ISSN 0098-0331. DOI 10.1007/BF02040093. [ Links ]
PEÑA-NEIRA, A., HERNÁNDEZ, T., GARCÍA-VALLEJO, M.C., CADAHIA, E., SIMÓN, B.F.D. y SUÁREZ, J.A., 1999. Low Molecular Weight Phenols in Cork Stoppers. American Journal of Enology and Viticulture [en línea], vol. 50, no. 3, pp. 285-290. [Consulta: 21/09/2020]. ISSN 0002-9254. Disponible en: Disponible en: https://www.ajevonline.org/content/50/3/285 . [ Links ]
PÉREZ-HERNÁNDEZ, L.M., CHÁVEZ-QUIROZ, K., MEDINA-JUÁREZ, L.A., GÁMEZ-MEZA, N., 2013. Compuestos fenólicos, melanoidinas y actividad antioxidante de café verde y procesado de las especies Coffea arabica y Coffea canéfora. BIOtecnia, vol. 15 no. 1. 51. Disponible en: https://biotecnia.unison.mx/index.php/biotecnia/article/view/136 [ Links ]
RODRÍGUEZ, R., et al., 1983. Flora arbórea de Chile [en línea]. S.l.: Editorial de la Universidad de Concepción. Disponible en: https://books.google.com.cu/books/about /Flora_arb%C3%B3rea_de_Chile.html?id=HrZgAAAAMAAJ&redir_esc=y . [ Links ]
ROZEMA, J., BJÖRN, L.O., BORNMAN, J.F., GABERSCIK, A., HÄDER, D.-P., TROST, T., GERM, M., KLISCH, M., GRÖNIGER, A., SINHA, R.P., LEBERT, M., HE, Y.-Y., BUFFONI-HALL, R., DE BAKKER, N.V.J., VAN DE STAAIJ, J. y MEIJKAMP, B.B., 2002. The role of UV-B radiation in aquatic and terrestrial ecosystems-an experimental and functional analysis of the evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology. B, Biology [en línea], vol. 66, no. 1, pp. 2-12. ISSN 1011-1344. DOI 10.1016/s1011-1344(01)00269-x. Disponible en: https://pubmed.ncbi.nlm.nih.gov/11849977/ . [ Links ]
SAXENA, M., SAXENA, J., PRADHAN, A., 2012. Flavonoids and phenolic acids as antioxidants in plants and human health. Int. J. Pharm. Sci. Rev. Res., vol 16 no. 2, 130-134. Disponible en: https://www.globalresearchonline.net/journalcontents/v16-2/28.pdf [ Links ]
SALMINEN, J-P., ROSLIN, T., KARONEN, M., SINKKONEN, J., PIHLAJA, K., y PULKKINEN, P., 2004. Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. Journal of Chemical Ecology , vol 30 no.9, 1693-1711. Disponible en: https://link.springer.com/article/10.1023/B:JOEC.0000042396.40756.b7 [ Links ]
SANTIBÁÑEZ, F., SANTIBÁÑEZ, P., GONZÁLEZ, P. 2016. Elaboración de una base digital del clima comunal de Chile: línea base (1980 2010) y proyección al año 2050. Informe final. Julio 2016. INFODEP. Ministerio del Medio Ambiente. Gobierno de Chile. p 99. Disponible en: http://basedigitaldelclima.mma.gob.cl/study/one [ Links ]
SHARMA, A., SHAHZAD, B., REHMAN, A. BHARDWAJ, R., LANDI, M. Y ZHENG, B. 2019. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules. Vol 24 no.13, 2452-2474. Disponible en: https://www.mdpi.com/1420-3049/24/13/2452 [ Links ]
SOLAR, A., COLARIÈ, M., USENIK, V. y STAMPAR, F., 2006. Seasonal variations of selected flavonoids, phenolic acids and quinones in annual shoots of common walnut (Juglans regia L.). Plant Science [en línea], vol. 170, no. 3, pp. 453-461. [Consulta: 21/09/2020]. ISSN 0168-9452. DOI 10.1016/j.plantsci.2005.09.012. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S0168945205003614 [ Links ]
STEFÃNESCU, B.E., SZABO, K., MOCAN, A., y CRIAN, G., 2019. Phenolic Compounds from Five Ericaceae Species Leaves and Their Related Bioavailability and Health Benefits. Molecules vol. 24 no. 11, 2046. Disponible en: https://www.mdpi.com/1420-3049/24/11/2046 [ Links ]
VANHOLME, R., DE MEESTER, B., RALPH, J., BOERJAN, W. 2019. Lignin biosynthesis and its integration into metabolism. Current Opinion in Biotechnology , vol. 56, 230-239. Disponible en: https://www.sciencedirect.com/science/article/abs/pii/S0958166918301435?via%3Dihub [ Links ]
VIKTOROVÁ, J., KUMAR, R., REHOROVÁ, K., HOANG, L., RUML, T., FIGUEROA, C.R., VALDENEGRO, M., FUENTES, L. 2020. Antimicrobial Activity of Extracts of Two Native Fruits of Chile: Arrayan (Luma apiculata) and Peumo (Cryptocarya alba). Antibiotics. Vol. 9 no. 8, 444. Disponible en: https://www.mdpi.com/2079-6382/9/8/444 [ Links ]
VILLARROEL, C., VÁSQUEZ, R., VILCHES, C., ARAVENA, C., BUSTOS, M. 2020. Reporte anual de la evolución del clima en Chile 2019. Dirección Meteorológica de Chile. 37p. Disponible en: https://climatologia.meteochile.gob.cl/application/publicaciones/documentoPdf/reporteEvolucionClima/reporteEvolucionClima2019.pdf [ Links ]
VOGEL, H., RAZMILIC, I., SAN MARTÍN, J., DOLL, U. y GONZÁLEZ, B., 2008. Plantas medicinales chilenas: experiencias de domesticación y cultivo de boldo, matico, bailahuén, canelo, peumo y maqui [en línea], 2008. S.l.: Editorial Universidad de Talca. ISBN 978-956-7059-91-1. Disponible en: https://books.google.com.cu/books/about /Plantas_medicinales_chilenas.html?id=GiDWXwAACAAJ&redir_esc=y . [ Links ]
WITZELL, J., GREF, R. y NÄSHOLM, T., 2003. Plant-part specific and temporal variation in phenolic compounds of boreal bilberry (Vaccinium myrtillus) plants. Biochemical Systematics and Ecology [en línea], vol. 31, no. 2, pp. 115-127. [Consulta: 21/09/2020]. ISSN 0305-1978. DOI 10.1016/S0305-1978(02)00141-2. Disponible en: Disponible en: http://www.sciencedirect.com/science/article/pii/S0305197802001412 . [ Links ]
ZOECKLEIN, B.W. y MACARRÓN, E.L., 2000. Análisis y producción de vino [en línea]. S.l.: Acribia. ISBN 978-84-200-0936-0. Disponible en: https://books.google.com.cu/books/about/An%C3%A1lisis_y_producci%C3%B3n_de_vino.html?id=OTwGAAAACAAJ&source=kp_book_description&redir_esc=y . [ Links ]
Received: September 02, 2020; Accepted: March 01, 2021











 text in
text in