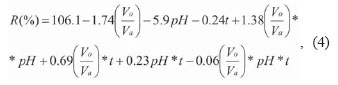Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Nucleus
versión impresa ISSN 0864-084X
Nucleus no.51 Ciudad de La Habana ene.-jun. 2012
CIENCIAS NUCLEARES
Evaluation of TBP, TOA and MEK as extractants to obtain ![]() radiotracers in organic phase from
radiotracers in organic phase from ![]() /
/![]() generator.
generator.
Evaluación del TBF, la TOA y la MEC como extrayentes para obtener radiotrazadores de ![]() en fase orgánica a partir del generador de
en fase orgánica a partir del generador de ![]() /
/![]()
Judith Domínguez Catasús1, Yusnier León Arias1, Regla Gamboa Marrero2, Aidamary Abreu Díaz1, Jorge Isaías Borroto Portela1
1 Instituto Superior de Tecnologías y Ciencias Aplicadas (InSTEC)
Ave. Luaces y Salvador Allende. La Habana, Cuba
2 Centro de Isótopos (CENTIS)
ABSTRACT
An alternative for radiotracer technique in Cuba, is the use of sodium pertechnate (![]() ) eluted from
) eluted from ![]() /
/![]() generator. This radioisotope generator is produced by the Cuban Center of Isotopes (CENTIS). Taking into account that solvent extraction is an usual procedure to separate
generator. This radioisotope generator is produced by the Cuban Center of Isotopes (CENTIS). Taking into account that solvent extraction is an usual procedure to separate ![]() from the fission products, this technique was used to get a radiotracer in organic phase from the eluted
from the fission products, this technique was used to get a radiotracer in organic phase from the eluted ![]() , using 30% TBP-16% TOA/ n-dodecane and 30% TBP-16% TOA/ ciclohexane as solvents. On the other hand, MEK is used as solvent in the so called
, using 30% TBP-16% TOA/ n-dodecane and 30% TBP-16% TOA/ ciclohexane as solvents. On the other hand, MEK is used as solvent in the so called ![]() /
/![]() extraction radioisotope generator. Consequently, it was also evaluated. In this paper, the influence on the extraction degree, of the volumetric ratio between organic and aqueous phases, the time of phase shaking and the time elapsed between shaking-end and phase separation were studied. Furthermore, in the case of MEK, the influence of NaOH concentration in the aqueous phase was considered. For the extraction using 30% TBP-16% TOA/ciclohexane and 30% TBP-16% TOA/n-dodecane, the influence of pH was also studied. The extraction degree of
extraction radioisotope generator. Consequently, it was also evaluated. In this paper, the influence on the extraction degree, of the volumetric ratio between organic and aqueous phases, the time of phase shaking and the time elapsed between shaking-end and phase separation were studied. Furthermore, in the case of MEK, the influence of NaOH concentration in the aqueous phase was considered. For the extraction using 30% TBP-16% TOA/ciclohexane and 30% TBP-16% TOA/n-dodecane, the influence of pH was also studied. The extraction degree of ![]() eluted from generator using MEK, as well as 30% TBP-16% TOA in n-dodecane or ciclohexane, in adequate conditions, was higher than 99%, so, any of the tested solvents could be used to get a
eluted from generator using MEK, as well as 30% TBP-16% TOA in n-dodecane or ciclohexane, in adequate conditions, was higher than 99%, so, any of the tested solvents could be used to get a ![]() radiotracer in organic phase
radiotracer in organic phase
Palabras claves: fullerenes, atomic displacements, cross sections, radiation effects, Monte Carlo method, gamma radiation.
RESUMEN
Una alternativa para el empleo de la técnica de radiotrazadores en Cuba ha sido el uso del pertecnetato de sodio (![]() ) eluido del generador de
) eluido del generador de ![]() /
/ ![]() producido en el Centro de Isótopos. Teniendo en cuenta que la extracción se utiliza regularmente para separar al
producido en el Centro de Isótopos. Teniendo en cuenta que la extracción se utiliza regularmente para separar al ![]() de los productos de fisión, se evaluó este método para obtener un radiotrazador en fase orgánica a partir del
de los productos de fisión, se evaluó este método para obtener un radiotrazador en fase orgánica a partir del ![]() , empleando como solventes la mezcla 30% TBF-16% TOA en n-dodecano y en ciclohexano. Por otro lado, la metiletilcetona se utiliza como solvente en el generador radisotópico de extracción de
, empleando como solventes la mezcla 30% TBF-16% TOA en n-dodecano y en ciclohexano. Por otro lado, la metiletilcetona se utiliza como solvente en el generador radisotópico de extracción de ![]() /
/![]() , por lo que también se evaluó para estos fines. En el trabajo se estudió la influencia de la relación volumétrica de la fase acuosa y la orgánica, del tiempo de contacto en agitación y del tiempo de reposo antes de la separación de las fases, en el grado de extracción del
, por lo que también se evaluó para estos fines. En el trabajo se estudió la influencia de la relación volumétrica de la fase acuosa y la orgánica, del tiempo de contacto en agitación y del tiempo de reposo antes de la separación de las fases, en el grado de extracción del ![]() . En el caso de la metiletilcetona se evaluó además, la influencia de la concentración de NaOH en la fase acuosa y para la extracción, con 30% TBF-16% TOA en ciclohexano y en n-dodecano, la del pH. El grado de extracción del
. En el caso de la metiletilcetona se evaluó además, la influencia de la concentración de NaOH en la fase acuosa y para la extracción, con 30% TBF-16% TOA en ciclohexano y en n-dodecano, la del pH. El grado de extracción del ![]() eluido del generador empleando, ya sea la metiletlicetona o la mezcla 30% TBF-16% TOA en n-dodecano o ciclohexano, en condiciones adecuadas, fue superior al 99 %; por tanto, cualquiera de los solventes evaluados se puede utilizar para obtener radiotrazadores de
eluido del generador empleando, ya sea la metiletlicetona o la mezcla 30% TBF-16% TOA en n-dodecano o ciclohexano, en condiciones adecuadas, fue superior al 99 %; por tanto, cualquiera de los solventes evaluados se puede utilizar para obtener radiotrazadores de ![]() en fase orgánica.
en fase orgánica.
Key words: radiation gamma, desplazamientos atómicos, secciones eficaces, efectos de las radiaciones, método de Monte Carlo, radiación gamma.
INTRODUCTION
Studies which expand the potentialities of the ![]() eluted from
eluted from ![]() /
/![]() to label silts [1], surface waters in non-reducing [2,3,4] or reducing [5] environment have been carried out in our country. However, alternatives that allow the use of this isotope for labeling organic fluids have sofar not been explored yet. Therefore, applications related with organic phase tracing have not been done in Cuba.
to label silts [1], surface waters in non-reducing [2,3,4] or reducing [5] environment have been carried out in our country. However, alternatives that allow the use of this isotope for labeling organic fluids have sofar not been explored yet. Therefore, applications related with organic phase tracing have not been done in Cuba.
The application and development of nuclear energy goes by the treatment and confinement of its residuals, especially those with high activity which result from the re-processing of nuclear fuel. ![]() and 129I radionuclides represent the dominant portion of the nuclear wastes with radiological risk [6].
and 129I radionuclides represent the dominant portion of the nuclear wastes with radiological risk [6].
Selective extraction with organic solvents is a commonly used procedure to separate ![]() from the rest of the fission products, with the objective of concentrating it before its final disposition. Tri-n-butylphosphate (TBP) and tri-n-octylamine (TOA) are two of the most used, either in self-sufficient form, in a mixture with diluters or in their one mixture. In all of these studies, the shaking times are between 10 and 15 min. Different diluents are use, among them: ciclohexane and n-dodecane [7-11].
from the rest of the fission products, with the objective of concentrating it before its final disposition. Tri-n-butylphosphate (TBP) and tri-n-octylamine (TOA) are two of the most used, either in self-sufficient form, in a mixture with diluters or in their one mixture. In all of these studies, the shaking times are between 10 and 15 min. Different diluents are use, among them: ciclohexane and n-dodecane [7-11].
On the other hand, it is also known that methylethylketone (MEK) is used as solvent in the so called ![]() /
/![]() radioisotope generator, which is produced nowadays by the Indian Radiation and Isotopic Technology Association [12].
radioisotope generator, which is produced nowadays by the Indian Radiation and Isotopic Technology Association [12].
The aim of the present work was to evaluate the extraction conditions of ![]() , eluted from
, eluted from ![]() /
/![]() radioisotope generator, using TBP, TOA and MEK as extractants in order to get a
radioisotope generator, using TBP, TOA and MEK as extractants in order to get a ![]() radiotracer for organic fluids.
radiotracer for organic fluids.
MATERIALS AND METHODS
General procedure for the extraction experiments
The extraction behavior of ![]() , eluted from the
, eluted from the ![]() /
/![]() generator, was studied using MEK, 30% TBP-16% TOA/ n-dodecane and 30% TBP-16% TOA/ ciclohexane. The parameters were modified according to the goal of each experiment.
generator, was studied using MEK, 30% TBP-16% TOA/ n-dodecane and 30% TBP-16% TOA/ ciclohexane. The parameters were modified according to the goal of each experiment.
The aqueous and organic phases were shaken inside the 20 mL plastic syringe used as separator device. A German shaker ILMENAU - MLW was use and the shaking speed was kept constant. The volume of the aqueous phase was fixed at 3 mL, while the organic phase volume was modified.
After shaking and some resting time, three samples of 0.5 mL each were taken from aqueous phase and measured 3 times with NaI (Tl) detector joint to the ratemeter.
To evaluate the extraction yield, the extraction degree, R (%), was determined as:
Where A (cps/mL) and B (cps/mL) are the radioactive concentrations of aqueous phase after and before extraction, respectively.
Each experiment was replicated 3 times for error estimation. Some studies were carried out using experimental design; in these cases the first experiment of the plan was performed 3 times.
Extraction of 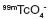 with MEK
with MEK
To study the influence on the extraction degree of the volumetric ratio of phases ![]() , NaOH concentration in the aqueous phase and time elapsed between shaking-end and phase separation (resting time), an experimental plan
, NaOH concentration in the aqueous phase and time elapsed between shaking-end and phase separation (resting time), an experimental plan ![]() was designed. R (%) was determined as the dependent parameter. Levels and modules (h) used for each parameter are shown in table 1. The experimental conditions were chosen taking into account previous experience [13].
was designed. R (%) was determined as the dependent parameter. Levels and modules (h) used for each parameter are shown in table 1. The experimental conditions were chosen taking into account previous experience [13].
Influence of shaking time on the extraction degree using MEK as solvent
Due to the short half life of ![]() (6.02 h), it is important to reduce as much as reasonable possible the time consumed in radiotracer preparation. So, the behavior of the extraction degree was studies at the following shaking times: 1, 2, 4, 6, 8 and 10 min. NaOH concentration in the aqueous phase was 5 M, the resting time was 10 min and Vo/Va = 1.
(6.02 h), it is important to reduce as much as reasonable possible the time consumed in radiotracer preparation. So, the behavior of the extraction degree was studies at the following shaking times: 1, 2, 4, 6, 8 and 10 min. NaOH concentration in the aqueous phase was 5 M, the resting time was 10 min and Vo/Va = 1.
Extraction of 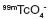 using 30%TBP-16%TOA/ ciclohexane and 30%TBP-16%TOA/ n-dodecane as solvents
using 30%TBP-16%TOA/ ciclohexane and 30%TBP-16%TOA/ n-dodecane as solvents
To study the influence on the extraction degree of the volumetric ratio of phases, pH in the aqueous phase and time elapsed between the end of shaking and phase separation, an experimental plan ![]() was designed. R (%) was again determined as dependent parameter. The aqueous solution pH was modified varying the concentration of
was designed. R (%) was again determined as dependent parameter. The aqueous solution pH was modified varying the concentration of ![]() . Table 2 shows the levels and modules (h) used for each parameter.
. Table 2 shows the levels and modules (h) used for each parameter.
Influence of pH on the extraction of 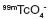 using 30% TBP-16% TOA in ciclohexane and n-dodecane as solvents.
using 30% TBP-16% TOA in ciclohexane and n-dodecane as solvents.
As the extraction mechanism of ![]() by TBP and TOA involves protons [7, 8, 16], the pH study was extended outside the performed experimental plans, (pH= 0.5 and 5). On the other hand, some points of the experimental designs were reproduced (pH=1, 2, 3 and 4) in order to validate the obtained mathematical models through the Kolmogorov Smirnov test. The shaking and resting times were fixed at 6 min and 10 min, respectively, and the phase volumetric relation was 1.
by TBP and TOA involves protons [7, 8, 16], the pH study was extended outside the performed experimental plans, (pH= 0.5 and 5). On the other hand, some points of the experimental designs were reproduced (pH=1, 2, 3 and 4) in order to validate the obtained mathematical models through the Kolmogorov Smirnov test. The shaking and resting times were fixed at 6 min and 10 min, respectively, and the phase volumetric relation was 1.
Influence of shaking time on the extraction of 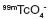 using 30% TBP-16% TOA in ciclohexane and n-dodecane as solvents.
using 30% TBP-16% TOA in ciclohexane and n-dodecane as solvents.
In this case, the extraction degree was determined after shaking 3, 6, 9, 12 and 15 min. The last one was the longest time reported in the consulted literature [11]. The resting time was 10 min, pH was fixed to 1, and Vo/Va = 1.
RESULTS AND DISCUSSION
Extraction of 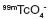 with MEK
with MEK
Results from the experimental plan are shown in table 3. Coefficients B1 (Vo/Va) and B2 (NaOH concentration) resulting from the analysis of experimental data are positive, quite similar and significantly higher than B3 (resting time before phases separation), which influence on the extraction degree is also positive.
The best result (99.76 ± 0.04) % was obtained when all parameters had been fixed at maximum levels. If the resting time is reduced to 5 min, the extraction degree just decreases in 0.87%. This behavior agrees with the results obtained by Karpeles and Rivero [13] using MEK to separate ![]() and
and ![]() from alkaline solutions. They found out the best extraction when NaOH concentration of aqueous solution was between 3 and 5 M. The design parameters were uncodified according to their modules and the model which describes its relationship with the extraction degree was established as (eq. 2):
from alkaline solutions. They found out the best extraction when NaOH concentration of aqueous solution was between 3 and 5 M. The design parameters were uncodified according to their modules and the model which describes its relationship with the extraction degree was established as (eq. 2):
MEK is a neutral extractant [14]. So, it is quite probably that extraction mechanism of ![]() will be the hidratation solvatation (eq.3), through the coordination water [15].
will be the hidratation solvatation (eq.3), through the coordination water [15].
Extraction of ![]() using 30% TBP-16% TOA/ ciclohexane and 30% TBP-16% TOA/ n-dodecane as solvents
using 30% TBP-16% TOA/ ciclohexane and 30% TBP-16% TOA/ n-dodecane as solvents
Table 4 shows results from the experimental design. All of the coefficients are statistically significant for a significance level of 0.05. The most important parameter is pH, which has a great negative influence in R (%). The lowest extraction degrees were obtained when pH was equal to 4 (experiments 3, 4, 7 and 8 from table 4). This perfectly matches with the roll of protons in the extraction mechanism for both extractants. Pruett et al. [7], El-Kot [16] and Kwan-Wook [8] stated that proton transferred by ![]() bonds to the phosphoric oxygen in TBP and
bonds to the phosphoric oxygen in TBP and ![]() joins to the proton as a counter ion.
joins to the proton as a counter ion.
On the other hand, TOA is a tertiary amine with a pair of non-shearing electrons at nitrogen atom, which participate in the extraction mechanism. In this case, besides the protonation mechanism, extraction goes by ionic exchange [8].
For both extractant systems, the phase volumetric relationship influences in a positive way but, its effect on the extraction degree is only important only for pH = 4. Even using the higher phase volumetric relationship (Vo/Va = 4) in experiments 2, 4, 6, and 6 from table 4, R(%) does not reach the values obtained when pH = 1 (exp. 1, 2, 5 and 6 ), no matter the phase of volumetric relationship used. It seems that as the total amount of active protons available for the extraction is smaller at pH = 4, the total amount of extractant molecules becomes a limiting factor.
In fact, from all over the coefficients that represent interactions between parameters, B12 (phase volumetric relationship-pH) is the highest, even a little bigger than the influence of phase volumetric relationship itself. When Vo/Va = 1 and pH = 4, the lowest extraction degree (91.4 ± 0.2) % was obtained.
As expected, the behavior of ![]() extraction using n-dodecane as diluent instead of ciclohexane is similar. Nevertheless, the first one could be considered a little more efficient under the worst conditions (pH = 4 and Vo/Va = 1), revealing a higher extraction degree ((95.93 ± 0.02) %).
extraction using n-dodecane as diluent instead of ciclohexane is similar. Nevertheless, the first one could be considered a little more efficient under the worst conditions (pH = 4 and Vo/Va = 1), revealing a higher extraction degree ((95.93 ± 0.02) %).
Finally, the extraction degree could reach (99.91 ± 0.01) % for ciclohexane and (99.86 ± 0.01) % for n-dodecane, if pH of aqueous solution is fixed to 1 with ![]() , shaking time is 15 min and resting time is 10 min.
, shaking time is 15 min and resting time is 10 min.
The parameters of the design were uncodified according to their modules and the model which describes its relationship with the extraction degree was established for 30% TBP-16% TOA/ciclohexane (eq. 4) and 30% TBP-16%TOA/n-dodecane (eq.5).
Influence of pH on the extraction of ![]() using 30% TBP-16% TOA in ciclohexane or n-dodecane as solvents.
using 30% TBP-16% TOA in ciclohexane or n-dodecane as solvents.
For 30% TBP-16% TOA/ciclohexane system (figure 1a), the experimental standard deviation varied between 0.001 and 0.036. When n-dodecane was used as a diluent (figure 1b), values ranged between 0.02 and 0.12.
For both systems, the extraction degree decreases from pH = 1 to pH = 5 in approximately 4%. Nevertheless, R (%) is always higher than 95%, which could be enough for some radiotracing applications.
No significant differences were observed in the extraction degree at pH = 0.5 and 1. This increase on NO3- concentration does not induce yet, any significant negative effect on ![]() extraction due to the competition of nitrates ions for TBP and TOA molecules [17, 18].
extraction due to the competition of nitrates ions for TBP and TOA molecules [17, 18].
The Kolmogorov Smirnov test, for 95% of probability, demonstrated that there are not significant differences between experimental data and models.
Influence of shaking time on the extraction degree
Figure 2 shows the behavior of the extraction degree versus the increase on shaking time, when MEK was used as solvent. From 6 min on, no more significant increase in R (%) is observed and the value is about 96.5%. This matches with experiment 7 (table 3) where Vo/Va was also 1. The standard deviations of the calculated extraction degrees oscillate between 0.05 and 0.20.
When using 30% TBP-16% TOA/ciclohexane, there are no significant changes on the extraction degree from 6 min on (figure 3a). The standard deviations ranged between 0.001 and 0.01. For 30% TBP-16% TOA/n-dodecane system (figure 3b), the standard deviations are in the same range, and the increase in the extraction degree from 6 to 15 min is just about 0.05%. So also in this case, 6 min of shaking time is enough for a successful extraction.
CONCLUSIONS
When MEK was used as solvent, the best degree of extraction was obtained by fixing NaOH concentration in aqueous phase to 5 M, volumetric relationship (Vo/Va) to 4, shaking time to 6 min and the resting time before phase separation to 10 min. The reduction of the resting time to 5 min provokes a diminishing of extraction degree just in a 0.87%. For 30% TBP-16% TOA/ ciclohexane and 30% TBP16% TOA/ n-dodecane solvents, the best results were obtained by fitting the aqueous phase pH to 1 with nitric acid, shaking the phases during 6 min and separating them after 10 min of rest.
The solvent extraction of ![]() , eluted from
, eluted from ![]() /
/![]() generator, using MEK and 30% TBP-16% TOA in n-dodecane or ciclohexane as solvents, could be used to get a radiotracer in organic phase for industrial applications. The selection of the solvent will depend on the nature of the system that will be traced and the cost-benefit analysis. Furthermore, it should be previously validated.
generator, using MEK and 30% TBP-16% TOA in n-dodecane or ciclohexane as solvents, could be used to get a radiotracer in organic phase for industrial applications. The selection of the solvent will depend on the nature of the system that will be traced and the cost-benefit analysis. Furthermore, it should be previously validated.
Acknowledgements
The authors would like to thank the IAEA for its partial financial support.
REFERENCES
1. BANDEIRA JV, BORROTO J. Development of a technique for using 99mTc as an adsorbable tracer for hydrodynamic studies of fine sediment in suspension. Appl Rad Isot. 2002; 57(1): 85-92.
2. BORROTO J, DOMÍNGUEZ J. Behaviour of 99mTc in highly polluted surface waters: Field and laboratory experiments. In: Isotope Techniques in Water Resources Development and Management. CSP-2/C. Vienna, 10-14 Mayo 1999.
3. DOMÍNGUEZ, J., BORROTO, J., HERNÁNDEZ, A. Empleo de trazadores en la obtención de modelos de calidad de agua del río Almendares. Nucleus. 2003; (34): 20-27.
4. DOMÍNGUEZ J, BORROTO J, PÉREZ E, HERNÁNDEZ A. Use of the 99mTcO4- and the Rhodamine-WT as Tracers and the Mathematical Convolution Procedure to Establish the Alarm Model In the Almendares River. Radioanal Nucl Chemistry. 2004; 260(2): 417-420.
5. DOMÍNGUEZ J, BORROTO J, GAMBOA R, et. al. Compuestos marcados en estudios hidrológicos. Memorias de la II Conferencia Internacional de Química. Universidad Central “Martha Abreu” de Las Villas. Santa Clara, Cuba. 2003. ISBN 959-250-080-0.
6. ASAKURA T. Technetium separation for future reprocessing. J. Nucl. Radiochem. Sci. 2005; 6(3): 271-274.
7. PRUETT DJ. The solvent extraction of heptavalent Technetium. Radiochimica Acta. 1981; 28(153).
8. KWANG-WOOK K. Extraction and stripping behavior of U-Np-Tc ternary system to TBP. J. Radioanal. Nucl Chem. 2002; 253(1): 3-10.
9. EIL-HEE L. Enhancement of Tc extraction and selective co-extraction of T, Np and U by adding a small amount of TOA in 30 % TBP/dodecane-HNO3 system. J. Korean Ind. Eng. Chem. 2001; 12(8): 883-889.
10. MAITI M, LAHIRI S. Separation of 99Mo and 99mTc by liquid-liquid extraction using TOA as extractant. J. Radioanal. Nucl. Chem. 2010; 283(3): 661-663.
11. FLORES A. Influence of extractant (TBP and TOA), diluents, and modifier on extraction equilibrium of monocarboxylic acids. J. Chem. Eng. Data. 2003; 48(4): 874-886.
12. Board of Radiation and Isotope Technology (BRIT). The 99Mo/99mTc Generator TCG-2. Department of Atomic Energy of India, 2008.
13. KARPELES A, RIVERO M. Obtención de soluciones de pertecnectato (Tc-99m) de alta concentración de actividad. República de Argentina. Comisión Nacional de Energía Atómica, 1973.
14. BRINGAS E. Contribución al Diseño de Procesos de Separación con Membranas Líquidas Selectivas. Tratamiento de Aguas Subterráneas Contaminadas con Cr(VI). [tesis doctoral]. Universidad de Cantabria, 2008.
15. NEFIODOV VD. Radioquímica. Moscú: Educación Superior, 1987.
16. EL KOT AM. Solvent extraction of heptavalent technetium. J. Radioanal. Nucl. Chemi, Artic. 1992; 163(2): 363-373.
17. PETERMAN DR, MINCHER BJ, et. al. Summary Report on Gamma Radiolysis of TBP/ndodecane in the Presence of Nitric Acid Using the Radiolysis/Hydrolysis Test Loop. Idaho National Laboratory Fuel Cycle Research & Development, 2010.
18. JASSIN TN. Effect of uranyl nitrat on the extraction of Pertechnic Acid by TBP solutions from nitric acids. Radiochim. Acta. 1983; 33: 163-167.
Recibido: 22 de marzo de 2012
Aceptado: 10 de mayo de 2012