My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.28 no.4 La Habana Sept.-Dec. 2011
REPORT
High level resistance to phytopathogenic fungi and oomycetes conferred by the use of a novel anti-microbial peptide
Inducción de alto nivel de resistencia a hongos fitopatógenos y oomicetos mediante el uso de un novedoso péptido antimicrobiano
Roxana Portieles1, Orlando Borrás1, Camilo Ayra1, Ernesto M González1, Juan Castillo2, Luis E Trujillo1, Osmani Chacón3, Mayra Rodríguez1, Yunior López1, Raisa Rodrígues1, Araiz Gallo1, Merardo Pujol1, Gil Enríquez1, Carlos Borroto1, Osvaldo Oliva1, Margarita Simón1
1División de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. La Habana, Cuba.
2Instituto Nacional de Ciencias Agrícolas, INCA. Cuba.
3Instituto de Investigaciones del Tabaco, IIT. Cuba.
ABSTRACT
Fungi and oomycete-borne plant disease constitute the largest limiting factor for crop productivity and yields both in Cuba and worldwide. Vast amounts of fungicidal chemicals have to be spent every year for their control, despite considerable environmental damage and the risk such chemicals pose for human health. Searching for biotechnological disease control alternatives is, therefore, a priority of current research on this subject. Plant defensins are small cysteine-rich peptides forming part of the defense mechanisms of plants that inhibit the growth of a wide range of phytopathogenic microorganisms. The present work describes a new cDNA coding for a defensin, isolated by looking at genes induced by the inoculation of Nicotiana megalosiphon with Peronospora hyoscyami f. sp. tabacina, the causative pathogen of tobacco blue mould. NmDef02, the isolated gene, was expressed in the yeast Pichia pastoris, and the purified recombinant protein exhibited a strong anti-microbial activity to major fungal and oomycetal plant pathogens. In addition, the constitutive expression of the NmDef02 gene in transgenic tobacco and potato plants increased their resistance to economically important diseases.
Keywords: phytopathogenic fungi, oomycetes, anti-microbial peptide, plant defensin, transgenic tobacco, transgenic potato.
RESUMEN
Las enfermedades en las plantas causadas por hongos y oomicetos son el factor de mayor incidencia que limita la productividad de las cosechas en el mundo. Enormes cantidades de fungicidas químicos se emplean cada año para controlar estos agentes patógenos, a pesar del daño al medio ambiente y el riesgo para la salud humana. Por ello, la búsqueda de alternativas biotecnológicas para controlar esas enfermedades es una prioridad. Una de las estrategias consiste en el uso de las defensinas: pequeños péptidos, ricos en cisteína, que forman parte del sistema de defensa de las plantas e inhiben el crecimiento de una amplia variedad de microorganismos fitopatógenos. En este trabajo se describe un nuevo ADN codificante para una defensina, aislado mediante pesquisa de genes inducidos por inoculación de Nicotiana megalosiphon con Peronospora hyoscyami f. sp. tabacina (el agente causal del moho azul del tabaco). El gen aislado, denominado NmDef02, se expresó en la levadura Pichia pastoris y su correspondiente proteína recombinante, que una vez purificada, mostró una potente actividad contra los principales agentes patógenos fúngicos y oomicetos. Además, la expresión constitutiva del gen NmDef02 en plantas transgénicas de tabaco y papa incrementó su resistencia a enfermedades vegetales de importancia económica.
Palabras clave: hongos fitopatógenos, oomicetos, pétido antimicrobiano, defensina de plantas, tabaco transgénico, papa transgénica.
INTRODUCTION
Plant defensins are a family of small, basic, highly stable cysteine-rich peptides typically 45 to 54 aminoacids long, distributed throughout the plant kingdom. These peptides are major players of the natural defense systems of vegetables, where they inhibit the growth of a disparate range of pathogenic microorganisms [1].
The present work describes the isolation of gene NmDef02, coding for a new defensin from the wild, non-cultured tobacco species Nicotiana megalosiphon. This gene was obtained following a strategy aimed at the obtention of an anti-microbial peptide able to confer high levels of protection against plant pathogens, comprised of two stages: 1) Expression in recombinant yeast, purification and in vitro evaluation of the anti-microbial activity of defensin NmDef02; and 2) Evaluation of the efficacy of the expression of this gene into transgenic tobacco and potato plants within the context of agricultural disease control [2].
RESULTS
Expression in recombinant yeast, purification and in vitro evaluation of anti-microbial activity of defensin NmDef02
A suppression subtractive hybridization cDNA library from leaves of N. megalosiphon inoculated with Peronospora hyoscyami f. sp. tabacina was used to isolate a clone bearing a 219 sequence denominated NmDef02, exhibiting sequence homology with a plant defensin. The sequence would code for a polypeptide with a length of 73 aminoacids, including a 27 aminoacid leader peptide. By means of alignments with plant defensin sequences from public databases and publications, it was possible to determine that the leader peptide cleavage site is conserved among all 74 studied polypeptides. There is strict conservation for eight cysteine residues thought to be involved in disulphide bonding, an aromatic residue at position 11 and glycines in positions 13 and 14 (ordinates are relative to those of defensin Rs-AFP2). A highly conserved serine at position 8 is not, however, present in NmDef02. Even though NmDef02 clusters with this group of 74 plant defensins (62% bootstrap support), it is likely to belong to a new defensin subgroup because only a poor bootstrap support (19%) links this sequence to its closest homolog from Triticum aestivum.
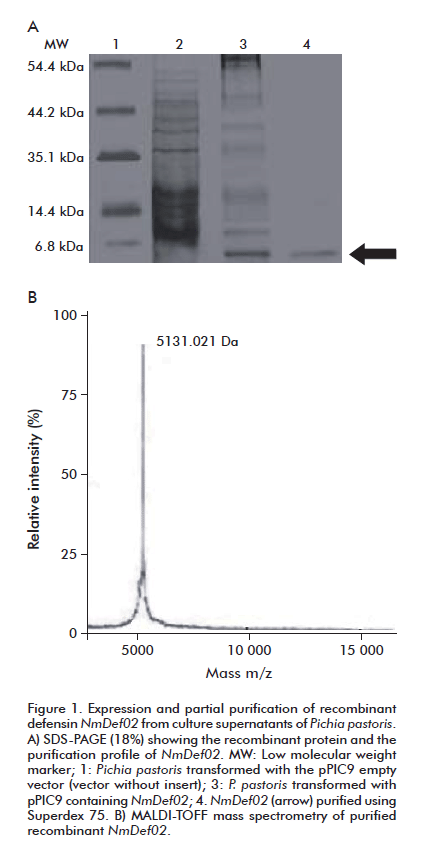
The cDNA sequence coding for the mature portion of NmDef02 was also fused in-frame with a modified α-factor leader peptide devoid of its last four aminoacids and transformed into the yeast Pichia pastoris. After inducing the expression of NmDef02 through the addition of methanol for 72 hours into shake flask cultures, it was possible to detect, by SDS-PAGE of culture supernatants, a protein species with an approximate molecular weight of 5.1 kDa, matching that of processed NmDef02 (Figure 1).
This recombinant species constitutes approximately 30% of all protein in culture supernatants, as estimated by densitometric analysis. It was not possible to detect this peptide in recombinant yeast transformed with the empty pPIC9 vector, used as negative control for the study. Recombinant NmDef02 was purified from culture supernatants, and the purified fraction was shown to be constituted by a single protein species of the expected size by mass spectrometry (MALDI-TOF).
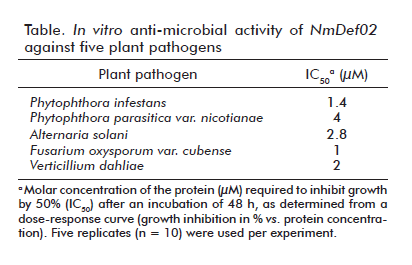
The anti-microbial activity of recombinant NmDef02 was evaluated in vitro on different plant pathogens. As shown in table, the recombinant molecule exhibits varying median growth inhibitory concentrations (IC50), ranging from 1 µM for Fusarium oxysporum var. cubense to 2.8 µM for A. solani after 48 hoursof incubation.
In the particular case of Phytophthora parasitica var. Nicotianae, recombinant NmDef02, at a concentration of 4 µM, was sufficient to inhibit hyphal growth after 48 hours of incubation. These experiments confirmed the efficacy and width of the in vitro anti-microbial activity of recombinant NmDef02, as it is able to inhibit the growth of both fungi and oomycetes.
Efficacy of the introduction of the defensin gene in transgenic tobacco and potato plants for the control of agriculturally significant diseases
Given that recombinant NmDef02 obtained from yeast exhibited anti-microbial activity in vitro against all five assayed plant pathogens, it was decided to generate transgenic tobacco and potato plants to determine whether expressing the 35S::NmDef02 transgene confers resistance against plant pathogens in vivo. Relative expression of the NmDef02 mRNA was measured before inoculation, by quantitative RT-PCR of 50 individual tobacco and potato lines homozygotic for the transgene. This followed the objective of identifying the best expressing clones for future research.
Although obtaining polyclonal antisera against NmDef02 would have allowed a more direct estimation of the levels of this molecule than measuring its transcript by quantitative RT-PCR, all efforts in this direction were fruitless, and therefore the latter technique was selected for this purpose. Not all tobacco and potato homozygotic lines, grown under greenhouse conditions, expressed the 35S::NmDef02 transgene at the same level, and transcript numbers were in general higher in tobacco than in potato plants. Twenty transgenic lines from each, having relatively high transcript levels of the NmDef02 cDNA, were chosen for further experimentation on resistance to pathogens under greenhouse and field conditions.
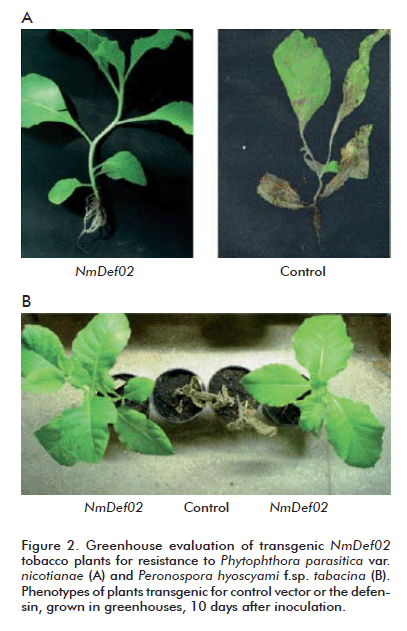
Twenty transgenic tobacco lines expressing the 35S::NmDef02 transgene, as well as control plants, were inoculated with the pathogenic oomycetes P. parasitica var. nicotianae or P. hyoscyami f.sp. tabacina (Figure 2). Five days after inoculation there were mild disease symptoms typical of P. parasitica var. nicotianae in the control plants, but the transgenic defensin-expressing plants remained healthy. Ten days after the inoculation there were severe disease symptoms in all control plants (stem rot and withering of the leaves), which died 5 days later. In the case of the transgenic plants expressing the 35S::NmDef02 gene, however, only 6.7% of the plants developed mild disease symptoms, while the remainder stayed healthy.
A similar effect was observed in transgenic tobacco lines inoculated with P. hyoscyami f.sp. tabacina. Transgenic plants expressing the defensin remained healthy 10 days after inoculation, whereas control plants died after the same period. Interestingly, there was a high correlation between relative NmDef02 transcription, as determined by quantitative RT-PCR, and resistance against P. parasitica var. nicotianae or P. hyoscyami f.sp. tabacina in several transgenic lines (data not shown). Lines 4, 5, 8, 28 y 49, expressing NmDef02 at relatively low levels, developed symptomatic infections.
There is strong evidence suggesting NmDef02 is involved in plant defense mechanisms, given the increased resistance of tobacco and potato transgenics expressing this defensin constitutively. The analysis of disease resistance of the transgenic lines of tobacco and potato evidenced that they were more resistant to P. parasitica var. nicotianae, P. hyoscyami f.sp. tabacina, A. solani and P. infestans, respectively than their wild-type counterparts or transformants containing solely the empty vector. Ours is the first report of high-level resistance of tobacco plants expressing NmDef02 to P. infestans, both under greenhouse and field conditions (Figure 3).
Both tobacco and potato transgenic plants remained healthy, without morphological changes or abnormalities even in cases where the accumulation of NmDef02 was high. The anti-microbial activity provided by the expression of this defensin was reached, therefore, without toxic effects on its plant hosts. Finally, it should be stressed that the present investigation further corroborates the efficacy of defensins in mediating resistance to agricultural disease, and points to these molecules as strong candidates for further work in the area of crop improvement.
REFERENCES
1. Portieles R, Ayra C, Borrás O. Basic insight on plant defensins. Biotecnol Apl. 2006;23:75-8.
2. Portieles R, Ayra C, Gonzalez E, Gallo A, Rodríguez R, Chacon O, et al. NmDef02, a novel antimicrobial gene isolated from Nicotiana megalosiphon confers high-level pathogen resistance under greenhouse and field conditions. Plant Biotechnol J. 2010;8(6):678-90.
Orlando Borrás. División de Plantas, Centro de Ingeniería Genética y Biotecnología, CIGB. La Habana, Cuba. E-mail: orlando.borras@cigb.edu.cu.