My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.31 no.3 La Habana July.-Sept. 2014
RESEARCH
Effect of a GnRH vaccine formulation on testosterone concentrations and reproduction in adult male rats
Efecto de una formulación vacunal basada en GnRH sobre la concentración de testosterona y la reproducción en ratas machos adultas
Franklin Fuentes1, Jesús Junco1, Lesvia Calzada1, Yovisleydis Lopez1, Eulogio Pimentel1, Roberto Basulto Baker1, Osvaldo Reyes Acosta2, Hilda E Garay-Pérez2, Gerardo Guillén-Nieto2
1 Centro de Ingeniería Genética y Biotecnología de Camagüey. Carretera Circunvalación Norte Apdo Postal 387, Camagüey, Cuba.
2 Centro de Ingeniería Genética y Biotecnología. Ave 31 e/ 158 y 190, Cubanacán, Playa. Apdo Postal 6072. CP. 10600, La Habana, Cuba.
ABSTRACT
In this study is described the effect of different doses of the gonadotropin releasing hormone (GnRH) mimetic peptide GnRHm1-TT, administered in three immunization schemes to define the most effective alternative combination for the treatment of prostate cancer. The design elements of the peptide immunogens are the GnRH-m1 linked to a T helper epitope of tetanus toxoid (TT) by chemical synthesis. Three doses of peptide GnRHm1-TT: 125, 300 and 750 µg were formulated in oil-based emulsions and tested in Copenhagen rats. Three immunization schemes (weekly, fortnightly and monthly) were used. As a result, the 750 µg dose generated a specific anti-GnRH antibody response in the fortnightly and monthly schemes, in contrast to 125 µg and 300 µg doses. The anti-GnRH seroconversion in the best responders corresponded with a decline, both in the testosterone levels down to castration and the size and weight of reproductive organs, an effect absent for the remaining doses of the three immunization schemes. The best variant corresponded to the 750 µg dose in a monthly regime. These findings demonstrated a marked immune-neutralization of the GnRH hormone for a subsequent immunological castration.
Keywords: cancer vaccine, GnRH, rats, castration, testosterone.
RESUMEN
Se describe el efecto de diferentes dosis y esquemas de inmunización de un péptido mimético basado en la hormona liberadora de gonadotropina (GnRH), con el objetivo de definir la variante más efectiva en el tratamiento del cáncer de próstata. Este péptido utilizado como inmunógeno, se sintetiza mediante la unión del decapéptido GnRHm1 con un epitopo del toxoide tetánico (GnRHm1-TT), por síntesis química. Fueron utilizadas tres dosis de tratamiento: 125, 300 and 750 µg, formuladas como emulsión oleosa y se evaluaron tres esquemas de tratamiento en ratas Copenhagen: semanal, quincenal y mensual. Como resultado, la dosis experimental de 750 µg generó una mayor respuesta humoral específica anti-GnRH al ser comparado con las restantes dosis, utilizando los esquemas de inmunización quincenal y mensual, y los mejores resultados en el último. Los niveles de seroconversión anti-GnRH de los mejores respondedores se correspondieron con la reducción de niveles de testosterona hasta niveles de castración y a la reducción máxima del peso y la talla de los organos reproductores. Estos hallazgos demostraron que hubo una inmuno-neutralización marcada de la hormona GnRH con la consiguiente castración inmunológica.
Palabras clave: vacuna contra cáncer, GnRH, ratas, castración, testosterona.
INTRODUCTION
Several treatment options exist for different stages of prostate cancer including observation, prostatectomy, radiation therapy, chemotherapy, and hormone therapy. Hormone therapy has evolved from the use of estrogens to gonadotropin-releasing hormone (GnRH) agonists and GnRH antagonists. GnRH analogs are widely used to block hypothalamic pituitary axis and therefore, represent the treatment of choice for prostate cancer patients. GnRH receptors are expressed in prostate cancer, even when the tumor has reached the castration resistant prostate cancer stage, and are endowed with antitumor activity, supporting the notion that they might be a molecular target for GnRH analog-based therapeutic strategies.
GnRH is a 10-amino acids peptide hormone which is secreted by the hypothalamus and transported by the hypothalamic hypophyseal portal system to the anterior pituitary. This hypothalamic peptide hormone controls the synthesis and secretion of sex steroid hormones (testosterone in males and estradiol and progesterone in females) and thus controls the whole reproductive function [1-3]. Testosterone is considered essential for the growth of prostate tumors. Within the prostate cells, testosterone is converted into 5-α-dihydrotestosterone (DHT), by the action of the 5-α reductase enzyme. As an intracellular androgen, DHT is approximately 10 times more powerful than testosterone. The production of the primary circulating androgen, testosterone, relies on the interplay of the hypothalamic-pituitary axis and the testes.
GnRH-based vaccines represent a promising anti-hormonal treatment alternative in prostate cancer, because these can reduce serum testosterone to castration levels. In turn, the ability to generate a memory immune response in vaccinated patients allows them to be without medication for relatively long periods of time, which also results in lower medication costs and marketing. This aspect gives vaccines high added-value and great competitiveness in the market [5].
An alternative approach to the use of GnRH analogs is the immunization with the native or a mimetic GnRH to induce anti-GnRH antibodies that may neutralize its biological activity, resulting in castration effects similar to those of GnRH drug therapy. In fact, immunization against GnRH has been shown to interrupt these biological activities in male mice [7].
GnRH immunogens have been reported to cause suppression in testicular activity in bovine for a long time [8, 9]. They can also be used to suppress reproductive functions in swine, as was the case of the GnRH dimmer in tandem conjugated to ovoalbumin (OVA) and emulsified in Specol [10]. This vaccine was highly immunogenic in healthy animals, inducing high anti-GnRH antibody titers and resulting in decreased testosterone levels, particularly significant in prostate and testicles weight reduction in pigs, dogs, and rats [11].
Futhermore, since GnRH is a short peptide, immunoenhancing approaches have to be implemented for successful vaccination. One the most common procedures to make a peptide immunogenic is to couple it to a carrier protein molecule, such as: KLH, TT, diphteria toxoid (DT), OVA, bovine and human serum albumin (BSA and HAS, respectively). Moreover, the origin of the carrier protein could be relevant for the conjugate immunogenicity levels, with heterologous proteins expected to result in conjugates of stronger immune responses. In general, several heterologous proteins can be used as carriers, but non-mammalian proteins tend to be highly immunogenic.
Additionally, the site of conjugation may determine the efficacy of the immunization [6]. The conjugation process used for the peptide-carrier fusion has caused losses during conjugation and made the goals [6]. Recently, multiple T-helper epitopes were chemically bound to GnRH to improve the immunogenicity and the castration levels in potential recipients [13]. It was shown that two immunizations with the G6k-GnRH tandem-dimer-OVA conjugate in a suitable adjuvant such as CoVaccine® HT causes a rapid and complete reduction of serum testosterone levels in sexually mature stallions [14]. It subsequently led to reduced sperm motility and affected testis function, while no adverse reactions were observed after immunizations. In a mouse model, it was demonstrated that anti-GnRH antibody responses can be induced by a synthetic GnRH3-hinge-MVP peptide. Mice treated with GnRH3-hinge-MVP-hsp65 had a significantly prolonged survival and suppression of local tumor growth, also showing reduced serum testosterone and luteinizing hormone levels (p < 0.05) [15].
The strategy was to induce immunity to GnRH by altering the target molecule on a synthetic peptide conjugated to TT immunogen (GnRHm1-TT) [16]. In this work, its immunogenic capacity and its correlation with a reduction in testosterone levels in a rat model was determined, using three doses and three immunization schemes. Our results are relevant for future clinical trials in prostate cancer patients.
MATERIALS AND METHODS
Peptide synthesis
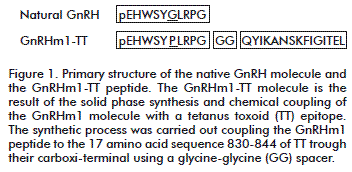
Peptides were synthesized on a solid phase using the Fmoc/tBu chemistry on Fmoc-AM-MBHA resin. Removal of the Fmoc group was carried out with 20 % of piperidine in Dimethylformamide, and the Fmoc-amino acids were coupled with DIC/HOBt activation. Cleavage from the resin and removal of side chain-protecting groups were accomplished by treatment with TFA/H2O/TIS (96.5/2.5/1) for 2 h, and the peptides were then precipitated with cold ether, dissolved in 40 % acetonitrile/water, and lyophilized [17]. Peptides were purified by RP-HPLC and identified by mass spectrometry (Figure 1).
Chromatography
Peptides were analyzed on an AKTA 100 (GE Healthcare USA, Piscataway, NJ, USA) HPLC system. Separation was achieved in an RP-C18 column (4.6 × 150 mm2, 5 μm) (Vydac, Grace, Deerfield, IL, USA), sith solvents: A (0.1 % of TFA in water) and B (0.05 % of TFA in acetonitrile). A linear gradient of 5-60 % of B for 35 min and a flow rate of 0.8 mL/min were used. Chromatograms were acquired at 226 nm, using the software package Unicorn 4.11 (GE Healthcare USA) for data processing of the RP-HPLC chromatograms. Peptides were purified on a LaChrom (Merck Hitachi, Darmstadt, Germany) HPLC system, using an RP-C18 column (Vydac, 25 × 250 mm2, 25 μm) and a linear gradient of 15-45 % of B for 50 min and a flow rate of 5 mL/min. Absorbance was monitored at 226 nm.
Animal models
Seventy-seven male Copenhagen rats of 8-9 weeks of age and females untreated for breeding were used. The animals were kept under a controlled environment at 20 ºC, 65 % mean humidity and a 14 h light/ 10 h dark photoperiod. Water and sterile feed access was ad libitum.
The animals were distributed at random in 11 experimental groups (7 rats each). Three doses of the GnRHm1-TT peptide were used: 125, 300 and 750 µg; and three schemes for each dose (weekly, fortnightly and monthly). Additionally, a group of castrated and placebo animals were used.
Immunogen preparation, immunization and mating
The GnRHm1-TT peptide was resuspended in phosphate buffered saline (PBS; v/v) and added, drop-by-drop, to the same volume of Montanide ISA 51 v/v. It was further shaked for 30 min to prepare the emulsion. Emulsions were prepared just before immunization.
Every scheme had rounds of 4 immunizations. The immunogens (500 µL) were administered subcutaneously on 4 sites along the supraescapular area, on both sides of the spine. The placebo animals were mated 4 weeks after the last vaccination with adult Copenhagen female rats. On the contrary, the males from the weekly scheme were mated after the last vaccination. The pairs (one per cage) stayed together for 2 weeks. Then, treated and untreated males were removed and anesthetized prior to sacrifice by cervical dislocation. The progenies from the pairs were registered and weighed.
Blood and serum collection and animal weighing
Blood was collected intraorbital puncture (500 μL approx.) and the animals were weighed, in all the schemes before each immunization, a week after the last immunization and on the day of sacrifice. The blood was centrifuged at 3200 rpm for 30 min, and sera collected and stored at –20 ºC until use.
Anti-GnRH antibody titer screening
The concentration of circulating GnRH-specific antibodies was determined by enzyme-linked-immunosorbent assay (ELISA). Solid-phase ELISA was performed using 96-well polystyrene plates (High binding, Nunc), coated with 10 μg/mL of natural GnRH peptide overnight at 4 ºC. Subsequently, plates were blocked with PBS (pH 7.4) supplemented with bovine serum albumin (BSA, 2 % v/v; Sigma) for 60 min at 37 ºC, and further washed three times with PBS-Tween 20 at 0.05 %. Then, the diluted serum samples were incubated 3 h at 37 ºC. After the corresponding washes, the anti-rat IgG-peroxidase conjugate was added to the working solution (1/8000) and plates were incubated for 60 min at 37 ºC. The reaction of anti-IgG antibodies against the GnRHm1 peptide was detected by adding orthophenylendamine (OPD) as chromogen and the H2O2 substrate dissolved in a proper buffer (dibasic sodium phosphate 0.02 M, pH 5) and incubated for 30 min at room temperature. Finally, the reaction was stopped by the addition of 2.5 N sulphuric acid. The plates were read at 492 nm with the microtiter plate reader (Multiscan, Labystem, Finland). Samples with absorbance values greater than the cut off line (0.159) were considered positive.
The immune response kinetics was calculated from the mean anti-GnRH antibody seroconversion for each experimental group. Sera were diluted 1/50, as the maximum absorbance value. The cut off value of the test (0.159) was calculated from a set of negative samples [18].
Testosterone determination
Testosterone levels were determined using the commercial TESTO CT2 kit, (CIS Bio International France). The sensitivity of the method, defined as the detectable concentration equivalent to twice the standard deviation of the zero-binding value, was aproximately 0.1 nmol/L, with a specificity of the test kit for testosterone higher than 99 %. Determinations were done in duplicates, by plating 25 μL of each serum sample directly in the pre-coated tubes, followed by incubation for 1 h at 37 ºC. Finally, the tubes were washed with distilled water and read in a gamma counter, with results expressed in nmol/L.
Macroscopic evaluation of reproductive organs
Animal testicles and prostates were removed and weighed on an analytical balance (Sartorius) and standardized against animals weights.
Statistical analysis
A multifactorial variance analysis was used, followed by a Duncan’s multiple range test (95 % confidence interval, p > 0.05).
The F ratio was set as 5.27844, calculated as the ratio between-groups estimate over the within-groups estimate, with estimates coming from the ANOVA variance data table. A p value lower than 0.05 was used for the F test, to fit the statistical differences between the means of the three variables at a 95 % confidence interval, and differences were determined by Multiple Range comparison test.
RESULTS
Anti-GnRH antibodies seroconversion
The means of anti-GnRH antibodies seroconvertion against the peptides used for immunization are shown in figure 2. The highest seroconversion values (absorbance at 492 nm) were obtained in the experimental group receiving 750 µg of the GnRHm1-TT peptide monthly and fornightly; whereas for the 300 µg dose in the three schemes, seroconversion values were discretely higher than the cut off line.
Testosterone levels
Regarding testosterone levels, it was observed that the 750 µg dose of GnRH m1-TT was the only one able to reduce testosterone values under castration levels (1.7 nmol/L) in the fortnightly and monthly immunization schemes, with the highest reduction in the monthly immunization scheme. Significant differences were achieved with this dose compared to the 125 μg (p = 0.0001), 300 μg (p = 0.0243) and placebo (p = 0.0013). Animals receiving 300 µg of the peptide only showed significant differences in testosterone levels in the monthly scheme, compared to those immunized with 125 µg (p = 0.021) and placebo (p = 0.0443) (Figure 3), but never reached castration levels. The immunization with 125 µg of GnRH m1-TT neither reduced testosterone levels below castration levels nor generated statistically significant differences with those of the placebo group.
Crossings
Fertility of immunized males was tested 15 days after the last GnRHm1-TT injection. Parallel studies were conducted with control males. The fertility data from pregnant rats are shown in the table. In the 750 μg dose groups in fortnightly and monthly immunization schemes), 1 out of 7 males (14 %) was fertile in both schemes. The rest of the experimental groups showed no significant differences from controls.
Post-mortem analysis of organs
The relation between testis and prostate weight and average GnRH binding percentage per animal is shown in figure 4. Significant differences were achieved for testicle weight between the dose and the immunization schemes used (p = 0.0007 and p = 0.0046, respectively; multifactorial variance analysis). Similar results were observed for prostate weight, with significant differences between doses and immunization schemes (p = 0.0012 and p = 0.0316, respectively). Testicle and prostate weight was significantly reduced only for the 750 μg dose compared to the placebo group, infact, highly significant for prostate weight in all the schemes (Duncan’s multiple range test). The 300 μg dose showed significant results only for prostate weight in the monthly scheme.
DISCUSSION
GnRH is a peptide showing a fully conserved structure throughout mammalian species, and serves as a homologous model for the human GnRH [4]. It normally joins the receptor of the hypophysis and causes the release of gonadotropin, necessary for testosterone production at the testicle level. This was the basis for studying the immunogenic capacity of GnRHm1-TT and the correlation with reduced testosterone levels in a rat model, which was determined by using three doses and three immunization schemes.
The active immunization against GnRH is one of the most prominent alternatives to surgical castration; however, the results from the studies made in some species, like swine and rats, have shown that the peptide antigen used which is similar to the endogenous GnRH was poorly immunogenic, an effect related with the haptenic nature of GnRH [19].
The administration of a GnRH-based formulation causes changes or damage in most of the organs of the reproductive system of mammals, markedly reducing the levels of circulating testosterone that leads to castration levels as the essential condition to achieve formulation efficiency [20].
Considering the natural tolerance to GnRH due to its conservation in mammals, a GnRH analogue variant was created with an aminoacid change of a glycine for a proline, and one T helper epitope from TT to increase the GnRH immunogenic capacity [16]. The different doses tested of the vaccine candidate in three different immunization schemes showed the best anti-GnRH antibody seroconversion results when administering 750 µg in a monthly scheme. In the groups receiving 125 µg and 300 µg doses antibody levels did not surpass the ELISA cut off line either the scheme. The phenomenon observed in these lower doses seems to correspond to the poor immunogenicity expected for an autologous, low molecular weight molecule like GnRH. Although it has been altered and coupled to an immunogenic carrier, it needs to be administered to attain antigen concentrations enough to mount effective immune activation, as determined by Talwar [4]. Alternatively, the low immunogenicity regarding all the doses in the weekly scheme seems to be related to a low maturation of the immune response, due to the huge antigenic load provided in terms of immunization frequency. Ultimately, the natural hormone is immunoneutralized by the generated anti-GnRH antibodies.
A direct correlation was observed between the anti-GnRH antibody seroconversion achieved with the 750 µg dose and the decrease in testosterone levels down to castration. Nevertheless, a similar effect was seen in the fortnightly scheme using the same dose, regardless the discrete anti-GnRH antibody seroconversion. In the weekly scheme, testosterone levels remained steady for any of the doses tested, in agreement with previous reports [4, 14, 20].
Post-mortem analysis of testicles and prostates of animals immunized with the 750 µg dose in the monthly scheme revealed a significant weight reduction of testicles and prostates compared to those of the control animals.
Additionally, there was a small, but significative decrease in prostate weight in the animals receiving the 750 µg and 300 µg doses in the fortnightly scheme; however, testicles weight did not varied the same [21]. No signs of biological effects in gonads and accessory glands were observed in the weekly scheme.
The weight decrease and atrophy in testicles and prostate of the animals immunized with GnRH modified variant suggest the entanglement of antibody-mediated GnRH neutralization, which subsequently deplete gonadotrophic hormones (luteinizing hormone and follicle-stimulating hormone) which play an important role in the normal development of the genital apparatus [22]. When the reproductive capacity of the animals in the different experimental groups was checked, the 750 µg dose in the fortnightly and monthly schemes induced an infertility process that may be caused mainly by a reduction in testosterone levels as essential hormone for spermatogenesis in males. These results greatly correlated with anti-GnRH antibodies, animal’s andrology and post-mortem analysis. It is suggested that reproductive organ atrophy would have caused of animal infertility.
There can be concluded that the best administration dose and immunization scheme combination is the 750 µg dose administered in a monthly scheme. This is a relevant result, considering it establishes the effective dose and immunization regime to develop a therapeutic vaccine against human hormone-dependent neoplasia, like prostate and breast cancer, of high incidence worldwide [23].
This vaccine candidate represents a promising anti-hormonal alternative in prostate cancer treatment. In turn, the potential to generate a memory immune response in vaccinated patients provides a therapeutic strategy without medications for relatively long periods of time which would also result in lower medication and marketing costs.
REFERENCES
1. Garner LL, Campbell GT, Blake CA. Luteinizing hormone (LH)-releasing hormone: chronic effects on LH and follicle-stimulating hormone cells and secretion in adult male rats. Endocrinology. 1990;126(2):992-1000.
2. Ghosh S, Jackson DC. Antigenic and immunogenic properties of totally synthetic peptide-based anti-fertility vaccines. Int Immunol. 1999;11(7):1103-10.
3. Jacobs E, Watson SA, Michaeli D, Ellis IO, Robertson JF. Anti-gonadotrophin releasing hormone antibodies inhibit the growth of MCF7 human breast cancer xenografts. Br J Cancer. 1999;80(3-4):352-9.
4. Talwar GP. Vaccines for control of fertility and hormone-dependent cancers. Immunol Cell Biol. 1997;75(2):184-9.
5. Trewartha D, Carter K. Advances in prostate cancer treatment. Nat Rev Drug Discov. 2013;12(11):823-4.
6. Ladd A, Tsong YY, Lok J, Thau RB. Active immunization against LHRH: I. Effects of conjugation site and dose. Am J Reprod Immunol. 1990;22(1-2):56-63.
7. Javid A, Ganaie M, Phil V, Shrivastava K. The effect of active immunization with gonadotropin releasing hormone conjugate (GnRH-BSA) ongonadosomatic indices (GSI) and sperm parameters in mice. Iranian J Reprod Med. 2008:6:119-23.
8. Cook RB, Popp JD, Kastelic JP, Robbins S, Harland R. The effects of active immunization against gnRH on testicular development, feedlot performance, and carcass characteristics of beef bulls. J Animal Sci. 2000;78(11):2778-83.
9. D’Occhio MJ, Aspden WJ, Trigg TE. Sustained testicular atrophy in bulls actively immunized against GnRH: potential to control carcase characteristics. Anim Reprod Sci. 2001;66(1-2):47-58.
10. Zeng XY, Turkstra JA, van de Wiel DF, Guo DZ, Liu XY, Meloen RH, et al. Active immunization against gonadotrophin-releasing hormone in Chinese male pigs. Reprod Domest Anim. 2001;36(2):101-5.
11. Basulto R, Milanes C, Rojas A, Fuentes F, Izquierdo N, Bertot JA. Effects of GnRH immunization in the testicular structure and function of adults dogs. Biotecnol Apl. 2003;20:20-4.
12. Junco JA, Basalto R, Fuentes F, Bover E, Reyes O, Pimentel E, et al. Gonadotrophin releasing hormone-based vaccine, an effective candidate for prostate cancer and other hormone-sensitive neoplasms. Adv Exp Med Biol. 2008;617:581-7.
13. Finstad CL, Wang CY, Kowalski J, Zhang M, Li ML, Li XM, et al. Synthetic luteinizing hormone releasing hormone (LHRH) vaccine for effective androgen deprivation and its application to prostate cancer immunotherapy. Vaccine. 2004;22(9-10):1300-13.
14. Turkstra JA, van der Meer FJ, Knaap J, Rottier PJ, Teerds KJ, Colenbrander B, et al. Effects of GnRH immunization in sexually mature pony stallions. Anim Reprod Sci. 2005;86(3-4):247-59.
15. Xu J, Zhu Z, Wu J, Liu W, Shen X, Zhang Y, et al. Immunization with a recombinant GnRH vaccine conjugated to heat shock protein 65 inhibits tumor growth in orthotopic prostate cancer mouse model. Cancer Lett. 2008;259(2):240-50.
16. Bringas R, Basulto R, Reyes O, de La Fuente J. Vaccine for the reversible immunocastration of mammals. Patent number: WO 9827111. 1998 Jun 25.
17. Fields GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int J Pept Protein Res. 1990;35(3):161-214.
18. Aguilar FF, Barranco JJ, Fuentes EB, Aguilera LC, Saez YL, Santana MD, et al. Very small size proteoliposomes (VSSP) and Montanide combination enhance the humoral immune response in a GnRH based vaccine directed to prostate cancer. Vaccine. 2012;30(46):6595-9.
19. Oonk HB, Turkstra JA, Schaaper WM, Erkens JH, Schuitemaker-de Weerd MH, van Nes A, et al. New GnRH-like peptide construct to optimize efficient immunocastration of male pigs by immunoneutralization of GnRH. Vaccine. 1998;16(11-12):1074-82.
20. Miller LA, Johns BE, Elias DJ, Crane KA. Comparative efficacy of two immunocontraceptive vaccines. Vaccine. 1997;15(17-18):1858-924.
21. Jayashankar R, Chaudhuri MK, Singh O, Alam A, Talwar GP. Semisynthetic anti-LHRH vaccine causing atrophy of the prostate. Prostate. 1989;14(1):3-117.
22. Jinshu X, Jingjing L, Duan P, Zheng Z, Ding M, Jie W, et al. A synthetic gonadotropin-releasing hormone (GnRH) vaccine for control of fertility and hormone dependent diseases without any adjuvant. Vaccine. 2005;23(40):4834-43.
23. Ornstein DK, Dahut WL, Liotta LA, Emmert-Buck MR. Review of AACR meeting: new research approaches in the prevention and cure of prostate cancer, 2-6 December 1998, Indian Wells, CA. Biochim Biophys Acta. 1999;1424(1):R11-9.
Received in April, 2014.
Accepted in December, 2014.
Franklin Fuentes. Centro de Ingeniería Genética y Biotecnología de Camagüey. Carretera Circunvalación Norte Apdo Postal 387, Camagüey, Cuba. E-mail: franklin.fuentes@cigb.edu.cu.