My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.32 no.4 La Habana Oct.-Dec. 2015
RESEARCH
Evaluation of morphological damage caused by a low molecular weight chitosan on the fungus Bipolaris oryzae
Evaluación de los daños morfológicos causados en el hongo Bipolaris oryzae por la aplicación de una quitosana de bajo peso molecular
Aida T Rodríguez-Pedroso1, Maribel Plascencia-Jatomea2, Silvia Bautista-Baños3, Mario O Cortez-Rocha2, Miguel Á Ramírez-Arrebato1
1 Unidad Científico Tecnológica de Base Los Palacios, Instituto Nacional de Ciencias Agrícolas, INCA. Carretera a Tapaste, Km 3 1/2, CP 32700, San José de las Lajas, La Habana, Cuba.
2 Departamento de Investigaciones y Posgrados en Alimentos, DIPA, Universidad de Sonora, Unison, México.
3 Centro de Desarrollo de Productos Bióticos, Ceprobi, Instituto Politécnico Nacional, IPN, México.
ABSTRACT
Antimicrobial activity of chitosan has been previously demonstrated on some fungi species. In this work, the direct effect of a low molecular weight chitosan (121.6 kDa) on the morphology of Bipolaris oryzae was studied by light and scanning electron microscopy techniques. Changes in the hyphae and germ tubes were observed. Moreover, structural and morphological changes during the apical growth phase of the fungus were detected by scanning elec-tron microscopy at 3 g/L chitosan. B. oryzae hyphae malformations were detected, with shortening and thickening of hyphae and spore germ tubes shortening and swelling, together with hyphae agglomerations, all these changes affecting the fungus’ multiplication and spreading capacity. These results support the possible use of the assayed chitosan in pest control strategies against B. oryzae in rice crops.
Keywords: germination, hyphae, scanning electron microscopy, rice, phytopathogen.
RESUMEN
La actividad antimicrobiana de las quitosanas ha ido demostrada en varias especies. En este estudio se evaluó el efecto directo de una quitosana de bajo peso molecular (121.6 kDa) sobre la morfología del hongo Bipolaris oryzae mediante cultivo in vitro y microscopía óptica y electrónica de barrido. Se detectaron cambios en las hifas y los tubos germinativos mediante microscopía óptica. Además, se observaron cambios estructurales y morfológicos durante la fase de crecimiento de crecimiento apical del hongo, cultivado en presencia de la quitosana a una concentración de 3 g/L. Se detectó la malformación de las hifas, con acortamiento y estrechamiento, así como acortamiento y engrosamiento de los tubos germinativos de las esporas, con aglomeración de las hifas resultantes, cambios que afectan la capacidad de multiplicación y diseminación del hongo. Estos resultados soportan la posible aplicación de la chitosana de 121.6 kDa para el control de las infestaciones por B. oryzae en los cultivos de arroz.
Palabras clave: germinación, hifas, microscopía electrónica de barrido, arroz, fitopatógeno.
INTRODUCTION
Chitosan has been found to be a valuable tool for the control of fitopathogenous fungi. It shows a variable response depending on the fungi species, and their spores seem to be more susceptible than hyphae to chitosan effects [1]. These last have been associated to several factors, such as: degree of deacetylation, molecular weight and concentration. It is known that the physiological and functional properties of chitosan depends on its molecular weight [2], with previous reports on the increase in the in vitro antifungal effect of chitosan on Botrytis cinerea as its molecular weight decreases [3]. Similar results have been also reported for Aspergillus niger and Penicillum expansum [4, 5].
Regarding the effect of chitosan on hyphae, this compound significantly compromise the morphology of Sphaeropsis sapinea, provoking the leakage, vacuolation and thinner hyphae [6]. Other morphological changes were observed in Rhizopus stolonifer hyphae at chitosan concentrations of 3 mg/mL [7]. On the contrary, this compound increased the average diameter of Aspergillus parasiticus spores and hyphae, reduced septation and raised the number of mitotic cell divisions that spores undergo during germination [8]. Scanning electron microscopy experiments [9] evidenced different alterations in Fusarium oxysporum f. sp. gladioli and R. stolonifer hyphae, such as: depression, swelling and distortion.
Therefore, due to the effects of chitosan on fungi hyphae, its potential application for the control of fungi infestations in economically relevant crops was investigated, particularly against Bipolaris oryzae (Breda de Haan) Shoem. This is a major pathogen affecting rice and the causal agent of the rice brown spot disease, its most significant damage in leaves, leading to the appearance of oval brownish spots with central gray areas and yellowish halo-like edges. During severe infestations, this pathogen causes leave withering before plants achieve their mature stage, also causing grain stains. In glumes, the spots are black or blackish-brown, covering the entire glume in severe infestations, ultimately leading to the partial or complete grain empty glumes.
Recently, chitosan was demonstrated to have an inhibitory effect on this pathogen [10], but the direct morphological manifestations caused by chitosan on this pathogen remained to be characterized. In this work, we characterized the morphological changes caused by low weight chitosan at a 3 g/L concentration on B. oryzae hyphae, by means of light and scanning electron microscopy observations (SEM), and on spore germination by SEM.
MATERIALS AND METHODS
Antifungal activity
A low molecular weight chitosan (121.6 kDa; Sigma-Aldrich) was used, with 90 % deacetylation. It was dissolved in a water solution of 1 % (v/v) and adjusted to pH 5.6 with 2 M potassium hydroxide [11]. The microbial strain was previously identified as Cochliobolus miyabeanus obtained from the fungi collection at the Laboratory of Plant Health of the National Institute for Forestry, Agriculture and Animal Research (INIFAP), Zacatepec, Morelos State, Mexico. The B. oryzae strain was grown in potato-dextrose-agar (PDA) culture media as recommended for this microorganism [10], in Petri dishes at 26-28 ºC for 7 days and under 12-h light/dark photoperiodicity. The chitosan solution and the culture media were sterilized separately in autoclave for 15 min at 121 ºC.
Afterwards, culture media were prepared containing chitosan at 1, 2, 3 or 4 g/L, respectively, and a control medium with no chitosan was also included. Then, 5 Petri dishes were prepared for each experimental condition, filled with 10 mL of each culture medium and further inoculated with 5-mm wide mycelial discs and grown under conditions as described. Colony diameters were measured manually every 24 h for 5 days and compared with the fungal growth in control medium devoid of chitosan, to calculate the colony radial growth and growth inhibition (%) by means of the equation:
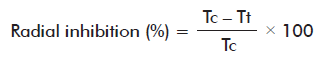
Where:
Tc: average colony radius in the control medium.
Tt: average colony radius in medium with chitosan.
Results of mycelial growth inhibition were analyzed by a completely randomized design with 5 replicas per treatment, ANOVA were established with the aid of the NCSS statistical software (NCSS LLC., version 2001) and mean values were compared with the Tukey’s multiple rank comparison test (p ≤ 0.05).
Light microscopy
Light microscopy images were captured after 72 h, when mycelium was grown on agar plates by visual scanning at 10× and 100× magnifications.
Spore germination
Ten microliters of a spore suspension at 1.4 × 105 spores/mL were seeded with a glass loop in Petri dishes filled with a thin PDA medium layer at 3 g/L chitosan, and further incubated at 27 ºC. Subsequently, 50 germinated and non-germinated spores, respectively, were counted under a light microscope (Olympus CX 31, Japan) at 40× magnification. Spores were regarded as germinated when the germ tube length was twice the overall diameter of the spore [12].
Scanning electron microscopy
B. oryzae cultures were established by disc inoculation and incubation, and its morphology was subsequently inspected by SEM during the deceleration of the fungi growth phase. Observations were made on 0.5 × 0.5 cm agar pieces taken 72 h after inoculation, sampled from either control or chitosan-containing culture medium. Samples were fixed by complete immersion in 5 % (v/v) glutaraldehyde solution at 4 ºC for 24 h, followed by a post-fixation step in 1 % (v/v) Osmium tetroxide solution at 4 ºC for 2 h. Subsequently, samples were progressively dehydrated by using a series of acetone concentrations (30, 40, 50, 70, 80, 90 and 100%, respectively) and further coated with gold particles prior to observations in SEM microscope (model JSM-5410 LV; Jeol Ltd., Japan).
RESULTS AND DISCUSSION
Results of the inhibition (%) of B. oryzae mycelial growth by chitosan are shown in figure 1. As expected, chitosan inhibited mycelial growth. The effect was directly proportional to the time of exposure and chitosan concentration as compared to the control medium culture results (p < 0.05), with the highest 97.2 % inhibition value attained at the highest concentration used (4 g/L) after 168 h of culture.
Our results are in agreement with previous reports on the inhibition by chitosan in other fungi species, particularly against Fusarium verticillioides with the same chitosan concentrations range tested [13]. Other groups found that a 133 and 147 kDa chitosans inhibited in 91.79 and 3.13 %, respectively, the radial growth in vitro of Ramularia cercosporelloides [14]. Otherwise, the same concentration of a 46.31 kDa chitosan caused a fungus growth until 2.81 ± 0.61 mm [15]. It is considered that the effect of the given chitosan on mycelial growth depends on the properties of the chitosan compound, attending to the source, molecular weight, deacetylation degree and the sensitivity to it of the fungi species tested [16].
The growth inhibition was found to coincide with morphological changes of the fungus under the light microscope. A more detailed study was made at the 3 g/L chitosan concentration, which altered mycelial growth in more than 50 %. A limited lateral growth was observed (Figure 2B) as compared with the control condition (Figure 2A), with a notable abnormal growth of the hyphae, characterized by hyphae swelling (Figure 2C). Similar results were reported in Fusarium f. sp. radicis-lycopersici, with increased hyphae diameter at 1 and 3 g/L chitosan concentrations, the highest with the last one [17].
Spores were also affected, as shown in figure 3. Their germ tubes developed an abnormal shape, with shortening and excessive swelling, together with hyphae agglomeration, when the fungus was cultured at 3 g/L chitosan. In this sense, another group found that 6 g/L chitosan, pH 5.6, was able to markedly reduce spore germination and shorten the germ tube elongation in B. cinerea and R. stolonifer to 90 and 75 % the normal values, respectively [18]. In fact, some studies mentioned that chitosan could be able to induce morphological changes in the fungus cell wall, and to reduce hyphae size and ramifications [6, 19]. Its mechanism of action could be related to the chitosan capacity to interact through its polycation surface positive charges with the negatively charged cell surface, further deorganizing it and losing its continuity what results in the leakage of cytoplasm contents, and ultimately causing structural and morphological changes [1].
Our SEM observations confirm that chitosan at the concentrations tested alter mycelial morphology (Figure 4B), producing deformations and distortions of the hyphae, instead of fungus cultured in the control medium devoid of chitosan which the characteristic unaltered mycelium (Figure 4A). This also coincides with previous findings in R. stolonifer and Alternaria alternata.
In summary, it was demonstrated that the in vitro culture of B. oryzae with a 121.6 kDa chitosan effectively inhibited mycelial growth, the more pronounced effect found in PDA medium containing 4 g/L chitosan, causing
morphological changes which were detected by light microscopy and SEM analysis after culturing in PDA medium containing 3 g/L chitosan. This could be the basis for the experimental use of this compound to inhibit the incidence of this phytopathogen in rice cultivars in vivo, since the changes detected are relevant to alter the fungus spread and multiplication capacity.
REFERENCES
1. de Oliveira Junior EN, Soares de Melo I, Teixeira Franco T. Changes in hyphal morphology due to chitosan treatment in some fungal species. Braz Arch Biol Technol. 2012;55(5):637-46.
2. Rabea EI, Badawy ME, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4(6):1457-65.
3. Badawy MEI, Rabea EI. Potential of the biopolymer chitosan with different molecular weights to control postharvest gray mold of tomato fruit. Postharvest Biol Technol. 2009;51(1):110-7.
4. Li XF, Feng XQ, Yang S, Wang TP, Su ZX. Effects of molecular weight and concentration of chitosan on antifungal activity against Aspergillus niger. Iran Polymer J. 2008;17(11):843-52.
5. Liu J, Tian S, Meng X, Xu Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol Technol. 2007;44(3):300-6.
6. Singh T, Vesentini D, Singh AP, Daniel G. Effect of chitosan on physiological, morphological, and ultrastructural characteristics of wood-degrading fungi. Int Biodeter Biodegr. 2008;62(2):116-24.
7. El Ghaouth A, Arul J, Asselin A, Benhamou N. Antifungal, activity of chitosan on post-harvest pathogens: induction of morphological and cytological alterations in Rhizopus stolonifer. Mycol Res. 1992;96(9):769-79.
8. Cota-Arriola O, Cortez-Rocha MO, Rosas-Burgos EC, Burgos-Hernández A, López-Franco YL, Plascencia-Jatomea M. Antifungal effect of chitosan on the growth of Aspergillus parasiticus and production of aflotoxin B 1. Polymer Int. 2011;60:934-44.
9. Bautista-Baños S, Ramos-García ML, Hernández-López M, Córdova-Albores L, López-Mora LI, Gutiérrez-Martínez P, et al. Use of scanning and transmission electron microscopy to identify morphological and cellular damage on phytopathogenic fungi due to natural products application. In: Mendez-Vilas A, editor. Current Microscopy Contributions to Advances in Science and Technology. Badajoz: Formatex; 2012. p. 401-5.
10. Rivero D, Cruz A, Martínez B, Ramírez MA, Rodríguez AT. Actividad antifúngica in vitro de las quitosanas K1 y Sigma frente a Bipolaris oryzae (B. de Haan) Shoem. Protección Vegetal. 2008;23(1):43-7.
11. Falcón-Rodríguez A, Costales D, Martínez MA, Gordon T. Respuesta enzimática y de crecimiento en una variedad comercial de tabaco (Nicotiana tabacum, L.) tratada por aspersión foliar de un polímero de quitosana. Cultivos Tropicales. 2012;33(1):65-70.
12. Paul GC, Kent CA, Thomas CR. Viability testing and characterization of germination of fungal spores by automatic image analysis. Biotechnol Bioeng. 1993;42(1):11-23.
13. Quintana-Obregón EA, Plascencia-Jatomea M, Sánchez-Mariñez RI, Rosas-Burgos EC, Cortez-Rocha MO. Inhibición del crecimiento micelial “in vitro” de la Fusarium verticillioides en presencia de quitosano. Rev Iberoamer Polímeros. 2010;11(6):386-91.
14. Quintana-Obregón EA, Plascencia-Jatomea M, Sánchez-Mariñez RI, Burgos-Hernandez A, González-Aguilar GA, Lizardi-Mendoza J, et al. Effects of middle-viscosity chitosan on Ramularia cercosporelloides. Crop Protection. 2011;30(1):88-90.
15. Quintana-Obregón EA, López-Cervantes J, Cira-Chávez LA, Sánchez-Machado D, Plascencia-Jatomea M, Cortez-Rocha MO. Actividad antifúngica del quitosano contra Alternaria tenuissima in vitro y en semilla de Cártamo. Rev Mex Fitopatol. 2011;29(2):168-71.
16. No HK, Meyers SP, Prinyawiwatkul W, Xu Z. Applications of chitosan for improvement of quality and shelf life of foods: a review. J Food Sci. 2007;72(5):R87-100.
17. Benhamou N. Ultraestructural and cytochemical aspects of chitosan on Fusarium oxysporum f.sp. radices-lycopersici, agent of tomato crown and root rot. Cytol Histol. 1992;82(10):1185-93.
18. El Ghaouth A, Arul J, Grenier J, Asselin A. Antifungal activity of chitosan on two postharvest pathogens of strawberry fruits. Phytopathology. 1992;82(4):398-402.
19. Sánchez-Domínguez D, Bautista-Baños S, Castillo-Ocampo P. Efecto del quitosano en el desarrollo y morfología de Alternaria alternata (Fr.:Fr.) Keissl. Anales Biol. 2007;29:23-32.
Received in February, 2015.
Accepted in December, 2015.
Aida T Rodríguez-Pedroso. Unidad Científico Tecnológica de Base Los Palacios, Instituto Nacional de Ciencias Agrícolas, INCA. Carretera a Tapaste, Km 3 1/2, CP 32700, San José de las Lajas, La Habana, Cuba. E-mail: atania@inca.edu.cu.














