INTRODUCTION
Life and career (Boutroux, 1903; Anonymous, 2023)
There is very little information about the life and education of Pierre Edmond Reboul. He was born on February 13, 1829, in Montpellier (Hérault) and passed away in Marseille on December 23, 1902. After receiving his Licence in physical and mathematics, he began his academic career as répetiteur and adjunct professor at the Lycée de Lille (1852) and then joined the Lycée de Chaumont as adjunct professor of physics (1853). He then moved to Pairs and worked as préparateur at the Faculty of Medicine in Paris, while working on his doctorate. In 1860 he received this degree from the Faculty of Science of Paris after successfully defending a thesis about ethers of glycide and their relation to the ethers of glycerin (Reboul, 1860). The examiners were Jean-Baptiste André Dumas (1800-1884), Antoine-Jerôme Balard (1802-1876), and Paul Quentin Desains (1817-1885). In 1861 was appointed assistant professor of at the Faculty of Sciences of Besançon, promoted to full professor in 1863, and in 1874 he was elected Dean of the Faculty of Sciences. In 1878 he was appointed professor chemistry at the Faculty of Sciences of Marseille and elected Dean of the Faculty in the same year, replacing Pierre Antoine Favre (1830-1880). In 1866 Reboul was elected corresponding member of the French Academy of Sciences and in 1869 he was appointed chevalier of the Legion d'Honneur in 1869 and promoted to officier in 1895. In 1874 the Academy of Sciences awarded him one quart of the Jecker prize (1,000 francs) for his work on the derivatives of glycerin, and in 1878, the full Jecker prize for his work on isomerism in the propylene series (Boutroux, 1903; Anonymous, 2020).
Scientific contribution
Reboul wrote about 40 papers and books (Reboul, 1893)4 mainly about organic chemistry. Care must be taken when reading Reboul's chemical formulas. In his early years he employed the old values of atomic masses, i.e., H = 1, O = 8, C = 6, so that ethanol was C4H5O2, diethyl ether C4H5-O2-C4H5, etc.
Glycerin derivatives
Glycerin was accidentally discovered in 1783 by Carl Wilhelm Scheele (1742-1786) while doing work on the similarities between soap and a drying plaster called emplastrum simplex, insoluble in water and partially soluble in concentrated alcohol (Scheele, 1783). In 1813 Michel Chevreul (1786-1889) reported that he had also isolated the sweet principle of Scheele, named it glycerin (from the Greek γλυκερός = sweet), and concluded that fats were composed of an anhydride of the sweet principle and an organic acid (Chevreul, 1815). In 1836 Théophile-Jules Pelouze (1807-1867) performed the elemental analysis of glycerin and found that it contained, by weight, 39.44% carbon, 8.73% hydrogen, and 51.83% oxygen, corresponding to the formula C6H16O6 (Pelouze, 1836). Finally, Marcellin Berthelot (1827-1907) established that glycerin was a polyalcohol of formula C3H5(OH)3, that it was to a monoalcohol as phosphoric acid was to a monobasic acid, and hence, capable of generating three esters: mono-, di-, and triglycerides while eliminating one, two, or three molecules of water (Berthelot, 1853,1854; Berthelot and Luca, 1855).
In the introduction to his thesis (Reboul, 1860) mentioned that glycerin could also react with the hydrogen halides and form the pertinent halohydrins, for example, monochlorohydrin, dichlorohydrin, and trichlorohydrin. The three bromohydrins were known but not so the chlorohydrins. Glycerin also reacted with alcohols to yield the pertinent mixed ethers or ethers with alcoholic radicals accompanied by elimination of water. This reaction was easily done using the pertinent alkyl halide, R-X. The three combinations ethylglycerin (ethoxyglycerin), C6H7(C4H5)O6, diethyl glycerin, C6H6(C4H5)2O6, and triethylglycerin, C6H5(C4H5)3O6, were known (see above remark about formula writing). According to Reboul, in theory it would be possible to synthesize glycerin derivatives in which one of the hydroxyl groups had combined with a hydrogen halide and another with an alcohol. In addition, there was another group of compounds remarkable because they were derived and the straight relations that they presented. These compounds could be derived from an oxygenized body, glycide, (probably from glycerin anhydride) C6H6O4, which had not been isolated, and which could be considered as the anhydride of glycerin C6H8O6, from which it differed by 2 equivalents of water, and to which it bear the same relation as lactide did to lactic acid and the amides to ammonium salts. Glycide would play the part of a diatomic alcohol and would yield three series of ethers represented by the general
C6H6O4 + A - 2HO
C6H6O4 + A + A' - 4HO
C6H6O4 + A + A' + A" - 6HO
Where A, A', and A" represented the formula of one equivalent of an alcohol or a monobasic acid (HO was the accepted formula for water). Hence, compounds existed, which were connected to an oxygenated body and could be regarded as ethers. The compounds of the first series (i.e., those with one equivalent of acid or alcohol) were derived from the second series of the glyceric ethers by the loss of one equivalent of a hydracid. The compounds of the second series were derived in the same manner form the glyceric ethers of the same series (Reboul, 1860).
Reacting glycerin dihydrochloride (dichlorohydrin) with an alkali resulted in the loss of HCl: C6H6Cl2O2 - HCl = C6H5ClO2. Replacing dichlorohydrin by the corresponding hydrobromide resulted in the formation of glycide bromohydride, C6H6Br2O2. It was clear that the compound chlorobromohydride resulted from the similar reaction, C6H5ClO2 + HBr = C6H6ClBrO2 and glycide acetochlorohydrin, from the reaction with acetic acid: C6H5ClO2 + C4H4O4 = C6H6(C4H3O4)ClO4. Glycide hydrochloride resulted from the reaction of dichlorohydrin with KOH: C6H6Cl2O2 - HCl = C6H5ClO2. This glycide served as the basis or the preparation of many of the compounds described in the thesis. Dichlorohydrin was easily prepared by saturating a mixture of glycerin and glacial acetic acid with gaseous HCl at 100 0C. The dichlorohydrin could be separated by fractional distillation (quite a long and hard process), or by heating the crude product with KOH. Reboul described glycide hydrochloride as a colorless liquid, heavier than water, smelling like chloroform, of density 1.194 at 11 0C, boiling at 1180-119 0C (atmospheric pressure), little soluble in water and completely in alcohol and ether. Elemental analysis indicated that it contained, by weight, 38.6% carbon, 5.4% hydrogen, and 38.6% oxygen, corresponding to the formula C6H5ClO2 (Reboul, 1860).
Chlorohydrin combined directly with fuming HCl to form dichlorohydrin, C6H4Cl2, which could also be prepared by reacting chlorohydrin with phosphorus pentachloride, an action, which gave place to the formation of trichlorohydrin,
C6H5ClO2 + PCl5 = C6 H5Cl3 + PCl3O2
and this, decomposed by KOH, lost HCl, and formed the new body C6H5Cl3 - HCl = C6H4Cl2.
Reboul went on to describe the synthesis of many new derivatives and their properties, among them, bromohydrins, iodohydrins, chlorobromohydrin, dibromohydrin, glyceric ethers composed of one equivalent of hydracid and one of oxacid, chloroamyl glycerin, chloroethyl glycerin, ethyl chlorohydrin, a chloramide, C12H12ClNO4, and a sulfurized derivative, C6H6S2O2 (Reboul, 1860).
The work of Reboul on the derivatives of glycerin earned him one quart of the Jecker Prize of the French Academy of Sciences for the year 1874.
Isomerism in the propylene series
Hydrocarbon halides
In 1860 Valérien Sawitsch reported that the decomposition of brominated ethylene bromide (CH4HBr,Br2) by an alcoholic solution of KOH resulted in the formation of HBr, which combined with the alkali, dibromoethylene, and a small amount of a highly volatile and inflammable compound, probably belonging to the acetylene series because its vapor produced a dark red precipitate with an ammonia solution of copper oxide (Sawitsch, 1860). This last fact led Reboul to study the reaction that generated this unidentified compound (Reboul, 1862a).
In a first experiment, Reboul added brominated ethylene bromide, drop-wise, to a boiling alcoholic KOH solution and noticed an abundant release of a gas, which he cleared of CO2 by passing it thorough KOH. Eighteen cm3 of bromide yielded about 1.5 L of a gas igniting spontaneously in contact with air and totally absorbable by an ammonia solution of silver nitrate or of cuprous chloride. This gas was found to be a mixture of acetylene and a new compound, gaseous at room temperature, which turned out to be a mixture of the brominated acetylenes C4H2Br2,Br2 and C4HBr3,Br2. Streaming this gas mixture through a layer of bromine over water resulted in the abundant formation of a crystalline product. Recrystallization from alcohol showed it to be a bromide of tribromoethylene, C4HBr3,Br3. This tribromide was a solid smelling like camphor, crystallizing as prisms melting between 400 and 50 0C (Reboul, 1862a).
According to Reboul, bubbling acetylene through bromine produced only the bromide C4H2Br2,Br2 and none of C4HBr3,Br2. The brominated dibromoethylene was easily prepared by the direct action of bromine on dibromoethylene (boiling at 88 0C). The product was a liquid having relative density 2.88 at 22 0C, insoluble in water and partially decomposing by distillation into vapors of bromine and HBr. Heating in a closed tube at 180 0C during several hours brominated tribromoethylene, alone or mixed with brominated dibromoethylene, bromine, and water, resulted in the formation of HBr and crystals of carbon sesquibromide. The bromide of tribromoethylene was little soluble in cold or boiling alcohol or ether and easily soluble in carbon disulfide. According to Reboul, perbrominated ethylene bromide, C4Br6, heated to 200 0C, decomposed into bromine and ethylene protobromide, C4Br4, fusible and volatile (Reboul, 1862a).
Reboul repeated that the reaction between brominated ethylene and an excess of boiling alcoholic KOH (as described above) provided dibromoethylene and a gaseous mixture of acetylene and brominated acetylene. Passing this mixture through washing flasks containing water, CO2, KOH, and cuprous chloride allowed obtaining pure acetylene in large quantities and in a short time. This gas reacted with bromine yielding C4H2Br4, accompanied by 6-8% of a crystallizable compound having a composition equivalent to the formula C4HBr3. Reboul concluded that his results indicated that under the influence of alcoholic KOH, brominated ethylene bromide yielded three different derivatives, originating from three different and simultaneous reactions: (1) ethylene dibromide, C4H2Br2, resulting from the elimination of one molecule of HBr, C4H3Br3 - HBr = C4H2Br2; (2) brominated acetylene originating from the elimination of a second molecule of HBr, C4H2Br2 - HBr = C4HBr; and (3) acetylene, produced by the simultaneous loss of HBr and Br2, C4H3Br3 - HBr - Br2 = C4H2 (Reboul, 1862b).
Reboul wrote that hydrocarbons of the general formula C2nH2n (alkenes) had the property of directly attaching 2 atoms of bromine to form the bromides C2nH2nBr2, which could lose one molecule of HBr to yield the monobromide derivatives of the primitive hydrocarbons, and another molecule of HBr to generate hydrocarbons of formula C2nH2n-2 (alkynes). Amylene (pentene) bromide also went through these series of reactions yielding C10H8, which Reboul named valerylene (actually, 1-pentyne, C5H8). Valerylene was a colorless and very mobile liquid, lighter than water, sparingly soluble in water, and having a penetrating garlic smell, boiling at 440 to 46 0C (at 745 mmHg). His formula indicated that it was the fourth term of the series C2nH2n-2. Valerylene was easily obtained by heating to 140 0C, in a closed tube, pentyl bromide with an alcoholic solution of KOH, adding water to the product, and separating the lighter phase, which contained a mixture of pentyne, alcohol, and amyl bromide. The alcohol was eliminated by water washes and the residue distilled to yield pentyne boiling at 440 to 46 0C. Pentyne reacted strongly with bromine yielding C10H8Br2, which began boiling at about 168 0C with decomposition (Reboul, 1864a).
A following paper described the bromides and hydrobromides of pentyne (Reboul, 1864b). It was well known that acetylene was able to add two bromine atoms to yield the bromide C4H2Br2, and Reboul had already shown that acetylene could also absorb four atoms of the halogen to become the tetrabromide C4H2Br4. These facts led Reboul to assume that all the members of the series C2nH2n-2 should show the same property; that is, they could absorb four bromine atoms. The experimental facts proved this assumption to be true: Pentyne reacted with bromine to yield the dibromide C10H8Br2 and the tetrabromide C10H8Br4, both liquid and isomers with dibromo and tetrabromobutene. The reaction with HBr also produced two hydrobromides isomeric with pentene mono- and dihydrobromide. The hydrobromides were prepared by reacting pentyne with a concentrated solution of HBr; the mixture heated up and became red colored. Upon addition of water the mixture separated into two liquid phases. The heavier phase was separated, washed with more water, and the two bromine derivatives separated by fractional distillation. The monobromide passed at 112 0C and the dibromide at the temperature 1700 to 175 0C (Reboul, 1864b).
The bromides were prepared by adding bromine drop-wise to pentyne cooled by ice. Two tetrabromides were produced: (1) C10H8""Br4, which remained liquid at -10 0C, and (2) (C10H7)""Br4, which crystallized as rhomboidal plates, very soluble in ether, and melted without decomposition. The dibromide C10H2Br2 could not be prepared by the direct action of bromine on pentyne. The only manner to prepare it was by distilling pentynyl bromide and collecting the fraction passing at 1600 to 172 0C (Reboul, 1864b).
Reboul reported (Reboul 1864c) that rectifying large amounts of crude monobromoamylene (monobromopentene) passed at 130 0C a fraction of purified monobromopentene and then, in 1700 to 190 0C, a new compound that after purification boiled at 1770 to 180 0C and had a composition corresponding the to the formula C14H13BrO = C10H8Br-O-C4H5. According to Reboul this compound resulted from the action of alcoholic KOH (potassium ethoxide) on the small amount of brominated monobromopentene that accompanied the bromopentene used in the preparation of brominated pentene 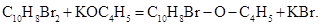 Heating this ether with alcoholic KOH to 1500-160 0C in a closed tube resulted in the loss of bromine as HBr in the formation of the new mixed ether
Heating this ether with alcoholic KOH to 1500-160 0C in a closed tube resulted in the loss of bromine as HBr in the formation of the new mixed ether 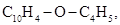 lighter than water, boiling at 1250 to 130 0C, and combining with bromine and iodine releasing HBr or HI (Reboul, 1864c).
lighter than water, boiling at 1250 to 130 0C, and combining with bromine and iodine releasing HBr or HI (Reboul, 1864c).
Reboul reported that heating crude bromopentyne (a mixture of di- and tetrabromide) with alcoholic potassium (potassium ethoxide) gave place to a strong reaction that destroyed the bromopentyne and generated many byproducts. Distillation of the same produced the following substances: (a) a liquid boiling about 1700-175 0C composed almost completely of dibromopentyne mixed with small amounts of the ether C10H8Br-O-C4H5, (b) brominated pentyne boiling about 1250-130 0C, and formula C10H7Br, and (c) a small quantity of a light liquid boiling about 450-50 0C, corresponding to a new hydrocarbon, which Reboul named valylène, C10H6, [2-methylbut-1-en-3-yne, HC≡C-C(CH3)=CH2, isopropenylacetylene]. Reboul assumed that the reaction went on as follows: Under the influence of KOH pentyne tetrabromide C10H8Br4 lost to atoms of bromine to become the dibromide, which was then destroyed in three different manners: (1) the alcoholic KOH extracted one-half or all its bromine as HBr and transformed it into bromopentyne and valylene,
C10H8Br2 - HBr = C10H7Br C10H8Br2 - 2HBr = C10H6
(2) extracted the two bromine atoms directly C10H8Br2 - 2Br = C10H8, or finally, (3) the elements of alcohol participated in the reaction and one or both bromine atoms disappeared and were replaced by one or two ethoxy groups (Reboul, 1865).
Afterwards, Reboul described the preparation and properties of bromopentyl, the complex cuprous valylene, valylene hydrochlorides, valylene mono- and dihydrochloride, valylene iodhydrate, pentyne mono- and diacetates, and of some of the polymers of pentyne (tripentyne, etc.) (Reboul, 1865, 1867a b).
It was easy to eliminate a molecule of HBr from ethylene bromide, boiling at 130 0C, and transform into brominated ethylene, C2H3Br. Inversely, this brominated ethylene could fix one molecule of HBr and become not ethylene bromide, but its isomer, brominated ethylene hydrobromide, boiling instead at 110 0C. Reboul was surprised that when repeating this synthesis, he obtained ethylene bromide instead of brominated ethylene hydrobromide (Reboul, 1870a). He realized that depending on the conditions, it was possible to obtain one or the other derivative: (1) HBr in a highly concentrated aqueous solution, at low or high temperature, transformed brominated ethylene into ethylene bromide. In lower concentration it always gave, by direct addition, brominated ethylene hydrobromide, a colorless compound, of pleasant odor, not freezing even at -18 0C, and of relative density 2.129 at 10 0C (against 2.198 for ethylene bromide at the same temperature). Alcoholic KOH transformed it into brominated ethylene. Reboul wrote that brominated propylene behaved in the same manner. Brominated propylene hydrobromide was a colorless liquid, boiling at 122 0C (740 mmHg) and relative density 1.955 at 9 0C. Alcoholic KOH transformed it into brominated propylene; (2) Heating a mixture of one equivalent of ethylene bromide and 2 equivalents of bromine, at 170 0C during several hours, allowed separating from the product of the reaction a certain amount of brominated ethylene bromide, C2H4Br.Br, presenting the same composition as ethylene bromide and brominated ethylene hydrobromide (Reboul, 1870a).
Reboul reported that HI caused the same phenomena, except that the production of the different isomers was a function of the temperature alone. On the contrary, HCl reacted with brominated ethylene to yield only brominated ethylene hydrochloride, and with brominated propylene only propylene chlorobromide. Brominated ethylene hydroiodide, C2H3Br.HI, was a heavy liquid of relative density 2.50 at 1 0C and boiling at 1410-142 0C (735 mmHg). Alcoholic KOH regenerated the brominated ethylene. Ethylene iodobromide, C2H4BrI, had relative density 2.70 at 1 0C and boiled at 160 0C with partial decomposition. Brominated propylene hydroiodide was a liquid of relative density 2.20 at 11 0C and boiling at 148 0C with partial decomposition into HI and brominated ethylene. Brominated ethylene hydrochloride, C2H3Br, HCl, was a colorless liquid of relative density 1.61 at 14 0C and boiling at 810-82 0C. Propylene chlorobromide was a liquid of relative density 1.62 at 16 0C, and boiling at 1120-113 0C without decomposition (Reboul, 1870b).
The next publication reported the results of the reaction between HBr and allyl bromide, isomeric with monobrominated propylene, prepared by the method of Bernhard Tollens (1841-1918) (Tollens, 1871). A concentrated solution of HBr transformed the allyl bromide into a mixture of two isomers, one distilling between 1430 and 145 0C and having all the properties of propylene bromide, and the second passing at 162 0C (Reboul, 1872a). Allyl bromide was prepared by bubbling gaseous HBr through allyl alcohol maintained in a glass balloon submerged in a bath of cold water. At the end of the process the liquid was present in two phases, an upper one containing allyl bromide, and a lower layer of aqueous allyl alcohol. The allyl bromide was separated, washed, dried, and purified by fractional distillation, yielding two products, one the known allyl bromide, the other another product boiling at 1620-164 0C, formed by a new isomer of propylene bromide. This compound had relative density 1.93 at 19 0C (Reboul, 1872a).
Reboul found that allylene (1-methylacetylene, 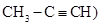 reacted rapidly with a concentrated aqueous solution of HBr at about 0 0C, yielding a mixture of mostly allylene dibromohydrate of formula CH3-CBr2-CH3, and a small amount of the monobromohydrate of formula CH3-CBr=CH2, easily separated by distillation. The dibromohydrate was a liquid boiling at 1140-115 0C, relative density 1.875 at 10 0C, and destroyed by alcoholic KOH into HBr and the monobromohydrate. The monobromohydrate was a liquid boiling at 480-490C (740 mmHg) and relative density 1.39 at 9 0C. It reacted with bromine yielding the dibromide CH3-CBr2-CH2Br, isomeric with brominated propylene bromide (Reboul, 1872b).
reacted rapidly with a concentrated aqueous solution of HBr at about 0 0C, yielding a mixture of mostly allylene dibromohydrate of formula CH3-CBr2-CH3, and a small amount of the monobromohydrate of formula CH3-CBr=CH2, easily separated by distillation. The dibromohydrate was a liquid boiling at 1140-115 0C, relative density 1.875 at 10 0C, and destroyed by alcoholic KOH into HBr and the monobromohydrate. The monobromohydrate was a liquid boiling at 480-490C (740 mmHg) and relative density 1.39 at 9 0C. It reacted with bromine yielding the dibromide CH3-CBr2-CH2Br, isomeric with brominated propylene bromide (Reboul, 1872b).
Allylene reacted also with HCl yielding the two chlorohydrates, with the dichlorohydrate present in the largest amount (Reboul, 1872b).
In five following papers Reboul demonstrated the bromohydrate and iodhydrate of brominated propylene were identical with the corresponding compounds of allylene (Reboul, 1872c) and described the preparation and properties of the different chlorides and chlorobromides of propylene (Reboul, 1873, 1874a), of normal propyl glycol and its esters (Reboul, 1874b), and of allyl- and diallyl acetic acids (Reboul, 1877). For example, normal propylene chloride, CH2Cl-CH2-CH2Cl (dichlorohydrin), was described as a liquid smelling like the Dutch liquid, CH2Cl-CH2Cl, boiling at 117 0C and relative density 1.201 at 15 0C. Alcoholic KOH transformed it first into allyl chloride, CH2=CH-CH2Cl, and then into ethylallyl ether, CH2=CH-CH2-O-C2H5 (Reboul, 1873). Of the four possible propylene chlorobromides the only one known was that of normal propylene, CH2Br-CHCl-CH3, which could be prepared by adding fuming HBr to allyl bromide. This substance was a heavy liquid of relative density 1.63 at 8 0C and boiling at 1400-141 0C (746 mmHg). Reboul described the preparation and properties of the three missing chlorobromides, CH3-CClBr-CH3, CH3-CH2-CHClBr, and CH2Br-CHCl-CH3 (Reboul, 1874a). Normal propyl glycol (1,3-propanediol), CH2OH-CH2-CH2OH, was a sweet, colorless, and thick liquid, of density 1.053 at 10 0C, boiling at 216 0C, and soluble in water and alcohol in all proportions. Its diacetate was prepared by reacting the corresponding dibromide with potassium acetate. The dibenzoate and divalerate were prepared by reacting the bromide with silver benzoate or valeric acid, while the chlorohydrins were synthesized by reacting the propyl glycol with HCl (Reboul, 1874b).
Pyrotartaric acid
Reboul reported that n-pyrotartaric acid (glutaric acid, 1,5-pentadioic) COOH-CH2-CH2-CH2-COOH) could be prepared by heating in a closed tube at 100 0C for three or four hours, n-propylene glycol dicyanide (CN-CH2-CH2-CH2-CN) with half its volume of aqueous concentrated HCl, followed by concentration on a water-bath and addition of absolute alcohol to separate the ammonium chloride (Reboul, 1876a). The alcoholic solution was evaporated to a thick brown syrup, which crystallized very slowly. This syrup was neutralized with baryta, and the excess of the reagent eliminated with a stream of CO2. The residue, after filtration and evaporation on a water bath, precipitated the pyrotartaric acid as its neutral barium salt, C5H6O4Ba+5H2O. This hydrate was very soluble in water and insoluble in alcohol and lost all its water at 135 0C. Pure normal pyrotartaric acid was obtained by treating the salt with diluted sulfuric acid. It melted at 96° to 97° and was very soluble in water, in alcohol, and in absolute ether (Reboul, 1876a).
According to Reboul, the current theories predicted the existence of four pyrotartaric acids C5H8O4, dibasic and isomeric, derived from pentane by substitution of two hydrogen atoms by two groups -COOH (Reboul, 1876b). Three of these acids were known: (a) ordinary pyrotartaric acid or propylene dicarbonic acid, (b) the ethylmalonic acid of Johannes Wislicenus (1835-1902) (Wislicenus, 1863), and (c) the dimethylmalonic acid of Wladimir Markownikoff (1838-1904) (Markownikoff, 1873). Reboul believed to be of interest to prepare and study the fourth acid, normal pyrotartaric acid or trimethyldicarbonic acid, starting from trimethylene bromide or normal propyl dibromide, Br-CH2-CH2-CH2-Br, which he had learned to prepare sometime before (Reboul, 1860, 1876b).
The synthesis of this acid was rather easy; it began by transforming propylene dibromide in its dicyanide by reacting one mole of the bromide with two of potassium cyanide, in the presence of alcohol. The resulting dicyanide was purified and converted into normal pyrotartaric acid as described in the previous publication (Reboul, 1877). The yield was 41 g of crystalline acid per 80 g of trimethylene bromide. This acid crystallized in triangular laminae belonging to the clinorhombic and hemihedral type. It distilled without alteration at 299° and melted at 96°. It was very soluble in cold water, 1 part in 1.2 part of water (at 14 0C) and in boiling water it dissolved in all proportions. It was also soluble in absolute ether and alcohol. Reboul described in detail the preparation and properties of its neutral barium, C5H6O4Ba+5H2O, and calcium salt, C5H6O4Ca+4H2O (Reboul, 1877).
Another publication described the preparation and properties of the pyrotartrates of zinc, C5H6O4Zn, copper, 2(C5H6O4Cu") +H2O, lead, C5H6O4Pb, silver, C5H6O4Ag2 neutral sodium C5H6O4Na2, acid sodium C5H6O4Na, ethyl [COO-C2H5-(CH2)3-COO-C2H5], and pyrotartaryl chloride, [COCl-C2H5,(CH2)3-COCl] (Reboul, 1876c). The zinc n-pyrotartrate was anhydrous, crystallizing as fine prismatic needles, little soluble in cold and boiling water. It could be prepared by saturating a cold aqueous solution of the acid with an excess of zinc carbonate, followed by concentration by evaporation. The silver salt was prepared by double decomposition of silver nitrate and sodium pyrotartrate. The latter salt was prepared by saturating with HCl a solution of normal acid. The ethylate was a colorless liquid, sparingly soluble in water, very soluble in alcohol, relative density 1.025 at 21 0C, and boiling at 235 0C without decomposition (Bourgoin and Reboul, 1877).
In 1877 Edme Alfred Bourgoin (1836-1897) reported that heating a mixture of ordinary pyrotartaric acid, bromine, and water, in a sealed tube for 26 hours at 132°C, produced CO2 accompanied by an odorous, colorless liquid of density 2.93, which gave off irritating vapors. Its elemental composition corresponded with the formula C2H2Br4, and its chemical and physical properties were the same as those of tribromoethylene hydrobromide (Bourgoin, 1877). Reboul and Bourgoin found that the composition of the product of the reaction of bromine with n-pyrotartaric acid varied with the temperature and amount of water present (Bourgoin and Reboul, 1877; Reboul and Bourgoin, 1877a). Thus, heating a mixture of 8.5 g of n-pyrotartaric acid, 21 g of bromine, and 10 cm3 of water, for two hours, in a closed tube at 145 0C, resulted in the complete absorption of bromine. Upon opening the tube, HBr was released accompanied by a mixture of CO and CO2. Reboul and Bourgoin were unable to determine the composition of the residual liquid; nevertheless, the result indicated that n-pyrotartaric acid was easily attacked by bromine. Treating two molecules of bromine with one molecule of n-pyrotartaric acid at 118-120 0C for nine hours in a sealed tube resulted in the formation of only CO2 and HBr. Upon cooling, the residual colorless and transparent liquid deposited an abundant precipitate of crystals little soluble in cold water and very soluble in boiling water, in ether, and in alcohol. Its properties and elemental composition corresponded to dibromosuccinic acid, showing that succinic acid and n-pyrotartaric acid were true homologues. According to Reboul and Bourgoin the reaction began by bromine reacting with n-pyrotartaric acid to yield pyrotartrate dibromide. The latter, under the oxidizing action of bromine in the presence of water, split into dibromosuccinic acid, CO2, and water. Heating 7.2 g of n-pyrotartaric acid with 18 g of bromine and 15 cm3 of water to 100° for 90 hours, gave some dibromosuccinic acid, dibromopyrotartaric acid and an oily liquid which, after purification by washing with caustic potash, crystallized from a mixture of alcohol and ether in crystals melting at 54-55 0C (Bourgoin and Reboul, 1877; Reboul and Bourgoin, 1877a).
In 1867 Bourgoin reported that the electrolysis of a concentrated solution of pure neutral potassium tartrate resulted in the liquor becoming alkaline at the negative pole, the release of a moderate amount of gas at both poles, and the solution at the positive pole remaining neutral, accompanied by a slow and continuous precipitation of potassium bitartrate (cream of tartar). The gas evolved at the positive pole was composed of CO2, oxygen, CO, and nitrogen. Most of the salt decomposition occurred at the positive pole (Bourgoin, 1867). The action of the current on a mixture of neutral tartrate and alkali produced results quite different from those obtained with neutral tartrate only, notwithstanding that the fundamental action was the same. A mixture of CO2, CO, oxygen, CO, and ethane was released at the positive pole. The decomposition of free tartaric acid yielded the same products as the neutral tartrate, though in different proportions, while acetic acid was formed at the positive pole (Bourgoin, 1867).
Reboul and Bourgoin found that n-pyrotartaric acid was very stable under the influence of an electrical current. A cold, almost saturated solution, released a very small amount of gas, even when the electrodes were positioned very close each other. Hydrogen was generated at the negative pole and oxygen at the positive one; the latter gas containing very small amounts of CO2 and CO. The acid became concentrated in the positive compartment of the cell and after two days deposited over the platinum strip small crystals of pyrotartaric acid. All these results indicated that pyrotartaric acid electrolyzed like mineral acids, i.e., sulfuric acid. Similarly, a neutral and concentrated solution of potassium pyrotartrate electrolyzed very easily, the positive compartment becoming full of hard acid crystals. The latter were found to be pure potassium acid pyrotartrate. After a long action of the electrical current the positive compartment became full of free pyrotartaric acid (Reboul and Bourgoin, 1877a b).
All these results indicated that under the influence of the electrical current the organic salt separated, as normally, into two parts, except that at the positive pole two simultaneous processes took place: (1) fixation of water on the elements of the anhydrous acid and regeneration of pyrotartaric acid, and (2) oxidation of a small amount of acid, according to the equation: (C5H6O3 +O) + 3O2 = 2CO2 + 3CO + 3H2O (Reboul and Bourgoin, 1877a b).
Reboul summarized all the above work in a lengthy memoir (58 pages) published in 1878, adding more information about the preparation methods and chemical analysis of the new compounds synthesized (Reboul, 1878). This paper was divided in three sections. The first one discussed the chlorides, bromides, and chlorobromides of propylene, and the new monochlorides and monobromides synthesized. The second part analyzed the derivatives of normal propylglycol and its dichloro and dibromo ethers. The last section analyzed normal pyrotartaric acid and its salts and its preparation by way of the reaction of normal propylene bromide with potassium cyanide.
The work of Reboul on the phenomena of isomerism in the propylene series earned him the Jecker Prize of the French Academy of Sciences for the year 1879.
Reaction with Amines
Reboul extended his work on the brominated propylenes to their reaction with tertiary amines (Reboul, 1881a b c d, 1882, 1883a b). In his first paper he discussed the reaction of triethylamine with CH3-CH=CHBr, a compound boiling at 60 0C (Reboul, 1881a) The reaction was carried at 100 0C in a closed tube, in the presence of an excess of the amine. The products of this reaction were triethylamine hydrobromide and allylene. The reaction was faster in the presence of an excess of absolute alcohol. The same results were obtained with the isomer CH3-CBr=CH2, which boiled at 48 0C. These two results indicated that triethylamine acted on these isomers in the same manner as KOH. The next isomer tested was allyl bromide, CH2Br-CH=CH2, which reacted on different manner yielding triethylallylammonium bromide, a white crystalline and deliquescent salt. Allyl chloride similarly but slower (Reboul, 1881a).
The action of heat upon triethylallylammonium bromide was found to be more complicated than that upon the bromides of quaternary ammonium (Reboul, 1881b). Heating the wet salt, in a flask connected to a gas receiver, it initially melted and then decomposed with a clear effervescence. The final residue was composed of two liquid layers, the lower one aqueous and alkaline, containing triethylallylammonium bromide triethylamine, diethylamine, and probably allylamine, and the upper one oily and strongly alkaline, and containing ethyl bromide and allyl bromide. The gas released was found to be ethylene, containing no traces of allylene. Very similar results were found when carrying the reaction in the presence of KOH, except that the oily phase seemed to also contain ethyl-allyl ether (Reboul, 1881b).
The paper described the reaction of triethylamine with isopropyl iodide (boiling at 910-92 0C) in a closed tube (Reboul, 1881c). At room temperature it was very slow and somewhat faster and 100 0C. As a result, it produced triethylamine hydroiodide, which crystallized, and propylene, as per the following scheme:
CH3-CHI-CH3 + N(C2H5)3 = N(C2H5)3,HI + CH3-CH=CH2
In the presence of absolute alcohol, the above reaction was accompanied by the formation of ethyl-isopropyl ether, boiling at 470-48 0C:
CH3-CHI-CH3 + C2H5OH = (CH3)2-CH-O-C2H5 + HI
The proportion among the different products varied with the temperature. At 150 0C the ether was the predominant product (Reboul, 1881c).
Reboul also reported the result of the reaction between triethylamine and tert-butyl bromide, (CH3)3C-Br (boiling at 730-74 0C), in a closed tube at 100 0C, to produce triethylamine hydrobromide and butylene. Once again, operating in the presence of absolute alcohol a second reaction took place generating the tert-butyl ethyl ether and HBr (Reboul, 1881c):
(CH3)3C-Br + C2H5OH = (CH3)3C-O-C2H5 + HBr
Triethylamine also reacted with epichlorohydrin to yield oxallyltriethylammonium chloride (Reboul, 1881d) and with symmetric trichlorohydrin, CH2Cl-CHCl-CH2Cl, to yield a mixture of triethylamine and the two isomers (- and (-chloroallyltriethyl ammonium chloride (Reboul, 1882). The reaction between diethylamine and epichlorohydrin yielded hydroxallyl tetraethyl diamine and HCl. This base was colorless and smelled like diethylamine; it was very stable and at atmospheric pressure it boiled at 160 0C without decomposition. It reacted with sulfuric and oxalic acids to give syrupy, neutral, and non-crystallizable salts. Treated with an excess of diluted HCl and then with chloroplatinate it gave a brick red chloroplatinate, crystallizing as prismatic rhomboids. (Reboul, 1883a b).
Isomerism in the butylene and acetylene series
Reboul did some preliminary work on both series (Reboul, 1889, 1891a; Reboul and Truchot, 1867). In his first work he reported the results on mixed butylic ethers (both groups butyl). The theory indicated that ten such ethers were possible, of which only two were known: the normal di-primary butyl ether of Adolf Lieben (1836-1914) and Antonio Rossi (Lieben and Rossi, 1869) and the bi-secondary ether of Friedrich Kessel (Kessel, 1878). In the first stage Reboul prepared the sodium derivatives of the four butanols, by boiling them under reflux with sodium in absence of air (Reboul, 1889). The process was easy with the first two primary and the secondary alcohols and very difficult with the tertiary one. These derivatives were decomposed very rapidly by water vapor and CO2 into the corresponding alcohol and NaOH more or less carbonated. Reboul used these alkoxides to prepare six of the ten possible mixed ethers. The reaction took place by double decomposition between a butyl bromide and a butoxide, generating the searched ether and sodium bromide. Normal di-primary butyl ether, [CH3-(CH2)3]2O, boiled at 141 0C and had relative density 0.784 at 0 0C; di-primary heminormal butyl ether, CH3-(CH2)3-O-CH2-CH(CH3)2, boiled at 131.50-132 0C at atmospheric pressure and had relative density 0.763 at 15.5 0C; primary-normal secondary butyl ether, CH3-(CH2)3-O-CH(CH3)(C2H5), boiled at 131.50-131.5 0C at atmospheric pressure and had relative density 0.7687 at 15 0C; and the primary normal-tertiary butyl ether, CH3-(CH2)3-O-CH(CH3)2, boiled at 1250-124 0C at atmospheric pressure; boiled at 100 0C with concentrated HBr it split into normal bromide and tertiary bromide (Reboul, 1889).
According to Reboul, of the four brominated butylenes known only one had an accepted structure, (CH3)2C=CHBr, obtained by elimination of HBr from isobutylene bromide. Reboul reported that he had been able to determine the structure of a second one by eliminating HBr from ethylene-ethylene bromide, CH3-CH2-CBr=CH2 (Reboul, 1891). This new compound was obtained by exposing to the sunlight a mixture of butyl bromide, bromine in the theoretical amount, and water, until complete discoloration. The lower layer of the final product was separated and distilled. The fraction passing above 160 0C was collected and found to contain ethylene-ethylene bromide (without isomers) and a monobrominated derivative of it. The latter boiled at 2180-224 0C at atmospheric pressure and decomposed releasing heavy fumes of HBr. The new butylene bromide was a colorless liquid smelling like garlic, boiling at 88 0C (759 mmHg) and having relative density 1.282 at 21 0C. Heated at 100 0C in a closed tube, in the presence of KOH and ethanol, it decomposed completely into HBr and ethyl acetylene, CH3-CH3(CH (Reboul, 1891).
Reboul and Pierre Truchot (1829-1887) remarked that parallel to the acetylene series there was another isomer one, formed by two molecules of acetylene. The first examples of this isomerism were hexoylene (hexyne) C6H10, the next higher homologue to valerylene, and diallyl (Reboul and Truchot, 1867). Heating hexylene bromide with alcoholic KOH yielded a mixture of brominated hexylene, C6H11Br, and hexylene, easily separable by distillation. Heating at 150 0C the separated hexylene bromide with alcoholic KOH yielded hexoylene and KBr. The substance smelled strongly like garlic, boiled at 760-80 0C, and had a relative density of 0.71 at 13 0C. Reboul also described the preparation and properties of brominated decylene bromide, C10H19Br, and decenylene (Reboul and Truchot, 1867).














