Meu SciELO
Serviços Personalizados
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Cubana de Ciencias Forestales
versão On-line ISSN 2310-3469
Rev CFORES vol.11 no.1 Pinar del Río jan.-abr. 2023 Epub 01-Abr-2023
Original article
Species of ectomycorrhizal fungi in two ecosystems of the Plan Café locality
1Universidad de Pinar del Río "Hermanos Saíz Montes de Oca". Pinar del Río, Cuba.
The study was carried out with the objective of identifying the species of ectomycorrhizal fungi in two ecosystems of the Plan Café locality. Dichotomous keys were used to identify the ectomycorrhizal species found. The following morphological characteristics were observed: shape of the cap, type of foot, type of blades, color and odor of the mushroom. Three edible species (Calvatia gigantea (Batsch) Lloyd, Agaricus campestris (L.), Agrocybe Sp), one toxic (Chlorophyllum molybdites (G. Mey.) Massee) and two of little culinary value due to their size (Lactocollybia sp, Tetrapyrgos nigripes (Fr.) E. Horak). These results suggest going deeper into the frequency and abundance of the identified species, as well as into bioecological aspects that serve as a basis for their use on the two farms under study.
Key words: Sheets; hat; cap; habitat; substrate.
INTRODUCTION
Fungi are microorganisms present in the soil, they participate in the decomposition of organic matter, the recycling of nutrients and the fungus-plant symbiosis. This relationship helps plants to obtain more quickly the water and nutrients that it needs, ensuring a good development of these (Delgado et al., 2019). Fungal species include mycorrhizae, which form a symbiotic association between plant roots and the mycelium of soil fungi (Salcido et al., 2020). These can be classified as endomycorrhizae and ectomycorrhizae; the latter are characterized by forming external mycelium and a Hartig network between the root cells without penetrating the cell wall (Salcido et al., 2020). There are more than 5,000 species of ectomycorrhizae that are mostly from the Basidiomycetes class, and around 3,000 species of angiosperms and gymnosperms with which they perform symbiosis (Martínez et al., 2016).
The absence of ectomycorrhizas affects the survival and development of plants, since they help the absorption of water and nutrients from the soil, increasing the tolerance of plants to acidity, heavy metal toxicity and high soil temperatures, as well as as it contributes to the resistance of diseases of the root system (Salcido et al., 2020). Hence the importance of considering them as a quality parameter of ecosystems, since they are common in moderately acid soils and rich in organic matter, both in boreal, temperate and tropical regions (Galindo-Flores., 2015, Montoya, 2016). This distribution being of great importance for the human being, since fungi are cosmopolitan organisms distributed in many regions of the world with a greater appearance in summer and autumn (Ramos and Mezas, 2017). Although the production and dispersal of fungi will be influenced by some climatic factors such as humidity, temperature, wind speed and atmospheric pressure (Ramos and Mezas, 2017). These organisms are important for humans because they contain components that contribute to its nutritional value, such as proteins, vitamins and minerals (Beltrán-Delgado et al., 2021). In addition, they present dietary fiber and an abundance of essential amino acids, as well as compounds of therapeutic interest, which include molecules such as polysaccharides (mainly -D-glucans), heteroglycans, chitin, peptidoglycans, proteoglycans, lectins and ribonucleic acid, lactones, phenols, terpenoids and alkaloids, antibiotics and metal chelating agents (Beltrán-Delgado et al., 2021).
Taking into account the above, the objective is to identify the species of ectomycorrhizal fungi in two ecosystems of the Plan Café locality.
MATERIALS AND METHODS
Description of the scenarios used in the investigation
The investigation took place in the town of Plan Café located at Km 4 of the Puerta de Golpe highway belonging to the municipality of Consolación del Sur in Pinar del Río. In the locality under study, two ecosystems located at 22°46'27 North latitude 83°56'51" West longitude were sampled. In them, the Ferralitic Yellowish Leached soil type predominates (Hernández et al., 2015), with a pH of 3.86; 7.04; respectively.
Sampling description
In the two ecosystems, a 1,000 m2, line was drawn. That allowed the sampling of five meters on each side of the former line to carry out the sampling in February, March and April, since at this stage the conditions are created for the appearance of the fruiting bodies, which were subsequently transferred to the microbiology laboratory for their macromorphological characterization.
Identification of ectomycorrhizal fungal species in silvopastoral systems
For the macromorphological study of the specimens, the methodology of Cifuentes et al. (1986) was mainly taken into account. The determination of the material was made through the use of dichotomous keys or guides García et al. (1998) and specialized sources such as (Dessing et al., 2000).
A census of the plants existing in the two ecosystems was carried out, taking their morphological characteristics to determine the species present.
RESULTS AND DISCUSSION
Table 1 shows that the two ecosystems evaluated present different species of plants, among which we can mention Roystonea regia Kunth, Dichrostachys cinerea , Acacia farnesiana Mill, Anacardium occidentale, Mangifera indica L, Ipomoea batatas; (L.) Lam, Zea mays L, Leucaena leucocephala Lam, Mimosa pudica L, Coffea arabica, Mangifera indica L, Citrus sinensis, Psidium guajava L, Samanea saman, Anacardium occidentale , Vigna unguiculata subspecies sesquipedalis, Morus alba L which are used in human and animal nutrition, gardening, living fences, construction, medicine and as wood (Table 1).
Table 1. - Description of the plant species in the ecosystems of the Plan Café locality
| ecosystems | Family | Gender | Species | Applications |
| E-1 | Arecaceae | Roystonea | Construction, medicinal and animal feeding | |
| E-1 | Fabaceae | Dichrostachys | Fences, construction, joinery and firewood for fuel | |
| E-1 | Fabaceae | Acacia | Gardening as ornamental, beekeeping, perfumery and to flavor ointments. | |
| E-1 | Anacardiaceae | anacardium | Feeding | |
| E-1 | Anacardiaceae | Mangifera | Wood, Food and Medicine | |
| E-1 | convolvulaceae | ipomoea | Feeding | |
| E-1 | Poaceae | Zea | Feeding | |
| E-1 | Fabaceae | leucaena | Animal feeding | |
| E-2 | Fabaceae | Mimosa | Medicine | |
| E-2 | Rubiaceae | coffee | Food, rituals, Industry | |
| E-2 | Anacardiaceae | Mangifera | Wood, Food and Medicine | |
| E-2 | Rutaceae | Citrus | Food, perfumery and | |
| E-2 | Myrtaceae | psidium | Food and medicine | |
| E-2 | Fabaceae | samanea | Shade, animal feed and alcohol production | |
| E-2 | Anacardiaceae | anacardium | Feeding | |
| E-2 | Fabaceae | Vigna | Feeding | |
| E-2 | moraceae | morus | Feeding |
Within these species, it can be established that the necessary requirements for the use of mangoes ensure food continuity through the contribution of vitamins and minerals present in the fruit (Salazar and Castro, 2022). In addition to the existence of other important species for consumption human and in construction, respectively, such as the Vigna unguiculata subspecies sesquipedalis which, in nutritional terms, are a great source of protein, as well as being rich in minerals (especially iron, zinc) and vitamins (Dago, et al., 2021). Samanea saman and Roystonea regia Kunth which according to (Mora et al., 2022) are used as wood due to the durability they have, determining it by the difference between the initial weight and the final weight (Table 2).
Table 2. - Abundance of plant species by ecosystems of the Plan Café locality
| Species | ecosystem 1 | ecosystem 2 |
|---|---|---|
| 0.09 | - | |
| 7.66 | - | |
| 0.03 | - | |
| 0.06 | - | |
| 0.14 | - | |
| 38.31 | - | |
| 53.63 | - | |
| 0.08 | - | |
| - | 0.29 | |
| arabica coffee | - | 28.54 |
| - | 0.29 | |
| - | 0.38 | |
| - | 2.85 | |
| - | 0.1 | |
| - | 0.57 | |
| - | 66.6 | |
| - | 0.38 |
Table 2 shows that the species with the highest abundance in the ecosystems were Ipomoea batatas; (L.) Lam, Zea mays L for one and Vigna unguiculata subspecies sesquipedalis, Coffea arabica for two, thus showing that these two ecosystems have the largest number of arable species for agricultural purposes. Coinciding with (MERAZ, 2019) which states that the corn plant makes an association with the mycorrhizae, thus improving the absorption of phosphorus and the total length of the root.
Identification of the fungal species found in the two ecosystems of the Plan Café locality
Fruiting body: 55 cm; attached to the soil by rhizomorphs.
Diagnostic gross feature
Large fruiting (can measure up to 65 cm), globe-shaped; sometimes laterally cleft, without a stem, with the base attached to the substratum by species of rootlets called rhizomorphs that tend to harden over time. The external surface (exoperidium) is usually smooth and white, breaking into irregular plates with maturation (a process called dehiscence), releasing the spores. The interior (gleba) is white and compact when young, becoming brown to yellowish-green and powdery when mature (Figure 1).
Nutritional mode: saprophytic
Substrate: on humus or on soil.
Habitat: forests, wooded gardens.
Fruiting season: summer, autumn.
Consumption: When it is young and white, in adulthood it is not consumed.
Kingdom: Fungi.
Division: Basidiomycota
Class: Agaricomycetes
Order: Agaricales
Family: Agaricaceae
Gender: calvatia
Species: Calvatia gigantea (Batsch) Lloyd.
This fungus presents a large fruit set that can measure up to 65 cm, globe-shaped and white in its youth, which presents a Saprophytic way of life for this species (Barroetaveña et al., 2020) . This species can be found solitary or in small groups on the ground (Verma et al., 2018).
Fruiting body: 5 - 10 cm in diameter and 7 cm in length.
Diagnostic gross feature: cap globose when young, flattening with maturity; white to whitish in color with slight pinkish-brown tones, with a fibrillose-fringed edge (Barroetaveña et al., 2020) .
Free, tight lamellae, whitish at first, rapidly pinkish, becoming almost black-brown with maturation. The foot is cylindrical and white, solid and without scales, easily separable from the cap that shares the same color. It has a simple, ascending whitish ring, which gradually detaches from the cap as it ripens, coinciding with the darkening of the lamellae (Figure 2).
Nutritional mode: saprophytic.
Substrate: on humus or on soil
Habitat: forests, gardens, woodlands, meadows
Fruiting season: fall and spring
Consumption: It is consumed at all stages of its life.
Kingdom: Fungi
Division: Basidiomycota
Class: Agaricomycetes
Order: Agaricales
Family: Agaricaceae
Genus: Agaricus
Species: Agaricus campestris (L.)
Álvarez et al 2021 found species of the genus Agaricus associated with the families Achatocarpaceae, Boraginaceae, Fabaceae, Nyctaginaceae and Polygonaceae presented during the dry and rainy seasons in Mexico. In addition, this genus is an obligate symbiont, so the absence of mycorrhizae affects the survival and development of the plants to which they are associated (Salcido et al., 2020).
Fruiting body: 100-180mm in diameter.
Diagnostic gross feature
The pileus -100-180mm- has little meat especially on the edge but it is firm. Initially, it can be spherical to oval, then hemispherical, and finally extended, tending to undulate or deform, somewhat depressed. Sometimes, it presents a slight obtuse ridge on the disc, not very evident. It may have scalloped margin, initially incurved, fibrous cuticle, non-separable, whitish, creamy color, with more or less pronounced pink tones. Depending on the degree of humidity, it darkens, taking on ocher or reddish tones, with handling or rubbing; with numerous large, flat plaques, brown or with darker reddish-brown tints in the center, on a cream background, detersile, which detach easily on the periphery, leaving only more adherent remains in the central part.
Blades, dense, with short and long lamellae; very broad, up to 15 mm wide; tight in the young and separated when old; cream-colored when young, they take on greenish tones with the maturation of the spores, ending from intense green to bluish-green, on a grayish background; arista slightly serrated, with colora.
Stipe, 80-120 x 10-15 mm; slender, fistulous; cylindrical, generally curved, slightly attenuated at the apex, and broadening at the base, subbulbose or bulbous; with shallow striations; at first white, darkening with age, handling or dry weather, until it takes on milky brown or reddish tones, darker in the grooves, somewhat lighter towards the apex.
Ring broad, membranous, double, at first sheathed and excess, later mobile; floppy in the upper part, formed by flat plates in the lower area; whitish above, brown or reddish-brown below.
Flesh, scanty, firm; at first white, it acquires ochraceous tints when cut, more intensely in the upper area of the crown, less pronounced the closer to the edge, in the stipe the color change is more homogeneous, only at the base are more reddish tones visible; pleasant smell, reminiscent of pastries (cupcake, biscuit); rafanoid flavor, somewhat spicy and bitter, this sensation disappears after a few seconds, leaving a certain radish nuance (Figure 3).
Nutritional mode: Saprophyte.
Substrate: grass, turf; in nitrified soils.
Habitat: parks and gardens, meadows, anthropized and ruderal areas (Becerra and Mateo 2018).
Fruiting season: fall and summer.
Consumption: toxic for human consumption.
Fungi kingdom.
Division: Basidiomycota.
Class: Agaricomycetes.
Order: Agaricales.
Family: Agaricaceae.
Genus: Chlorophyllum.
Species: Chlorophyllum molybdites (G. Mey.) Massee.
The species Chlorophyllum molybdites (G. Mey.) Massee presents a crown 100-180mm; little meat, especially on the edge, firm; initially spherical to oval, then hemispherical, and finally extended, tending to undulate or deform, somewhat depressed, sometimes with a slight obtuse ridge on the disc. In addition, this species has a ring and its mode of nutrition is Saprophytic (Becerra and Mateo 2018).
Next, we identify the genus Agrocybe which presents the following macromorphological characteristics.
Fruiting body: 10 and 40 mm in diameter.
Diagnostic gross feature
The cap is between 10 and 40 mm in diameter, broadly umbonate, initially bell-shaped, soon flat-convex and finally flat, with a slight central depression. Entire, regular margin, striated for transparency in wet weather, slightly involute in young specimens but extended when mature, when it may appear slightly lacerated. Cuticle smooth, somewhat rough, shiny, hygrophanous, slightly lubricated in humid weather, ochraceous brown in color at first, but with the passage of time or in dry weather, it becomes lighter to a light yellowish brown, almost white on the edge. Hymenium formed by sheets, lamellae and straight lamellules, from adnate to semi-free, initially yellowish-brown in color, gradually acquiring lilac tones, finally ending in dark brownish-grey. Spores in mass of ferruginous brown color.
Stem with a circular section, between 25-50 × 1.5-2.5 mm, thin, centered, fibrous, colored at the cap, almost whitish at the apex and darker at the base, covered with a fine whitish bloom. At the base it presents long whitish mycelial cords attached to 1 or up to 3 brownish-blackish sclerotia on the outside, whitish on the inside, which can reach up to 15 mm in diameter. Cream-colored meat, with insignificant odor and slightly astringent taste (Figure 4).
Nutritional mode: Saprophyte.
Substrate: Poplars, elms and other deciduous trees.
Habitat: Parks and gardens, meadows, forests.
Fruiting season: spring and autumn.
Consumption: Some species are edible for humans.
Fungi kingdom.
Division: Basidiomycota.
Class: Agaricomycetes.
Order: Agaricales.
Family: Strophariaceae.
Genre: Agrocybe.
Species: Agrocybe Sp.
The genus Agrocybe presents a fruiting body that ranges from 10 to 40 mm in diameter and with a saprophytic mode of nutrition (Aza et al., 2021). This genus sprouts profusely in large groups on stumps and trunks of poplars and other riverside trees which usually come out for a good part of the year but its early nature in the bud is known, fruiting already in August and without rain (Jiménez, 2022).
Fruiting body: 1 to 4 cm in diameter.
Diagnostic gross feature: the cap is 14 cm in diameter, convex-umbilicated throughout development, and funneled in old age. Tenacious, non-separable, hygrophanous cuticle, white in color, which yellows in old specimens (Hosen et al., 2016).
Lamellae and white lamellae tend to yellow over time, adherent or somewhat decurrent, tight and wide, compared to the thickness of the meat.
Flesh 1 mm thick in the center of the cap, white, hard, with a somewhat astringent flavor and a fungal odor, slightly spermatic, similar to that of some polyporaceas.
Stem 2-5 x 0.4-0.8 cm, central or somewhat eccentric, hollowed, flattened and furrowed, the same color as the cap (Figure 5).
Nutritional Mode: Saprophyte.
Substrate: Grassy, on wood and soil.
Habitat: Parks and gardens, meadows, forests.
Fruiting Season: Spring.
Consumption: Little culinary value for its size.
Fungi kingdom.
Division: Basidiomycota.
Class: Agaricomycetes.
Order: Agaricales.
Family: Marasmiaceae.
Genus: Lactocollybia.
Species: Lactocollybia sp.
The species of ectomycorrhizae belonging to the agarical order are related to angiosperm plants distributed in the tropical deciduous forest, which coincides with this specimen, which was found in association with Psidium guajava trees in tropical areas (Álvarez et al., 2021).
Fruiting body: 1 to 2 cm. diameter.
Diagnostic gross feature: This fungus has a crown: 1 to 2 cm. in diameter, convex to flat-convex. It presents a central to subinfundibuliform depression, with the margin slightly raised, furrowed-striated, surface glabrous, plicate-striated, greenish gray to white in color, sometimes dark colors in the center, reacts by turning dark blue when it is mistreated, it is not hygrophanous (López et al 2005).
Stipe: 1-4 cm long, 0.1 cm wide, fibrillar, cylindrical, without ring, also white towards the crown and black towards the base.
Lamellae: white to grey, smooth and subdistant. Spore present, white to cream.
Observations: this species is easily distinguishable by the greenish color of the crown and the dark color at the base of the stipe (Figure 6).
Nutritional mode: saprophytic.
Substrate: Soil, grass and decaying branches.
Habitat: meadows, gardens and pastures.
Fruiting season: spring and autumn.
Consumption: Little culinary value for its size.
Fungi kingdom.
Division: Basidiomycota.
Class: Agaricomycetes.
Order: Agaricales.
Family: Tricholomataceae.
Genus: Tetrapyrgos.
Species: Tetrapyrgos nigripes (Fr.) E. Horak.
Jagadish et al., (2019) found several species of ectomycorrhizal fungi associated with Anacardium occidentale, among which we can mention the species Tetrapyrgos nigripes, Agaricus sp. These species carry out a mycorrhizal symbiosis with the plant, helping it with nutrition by providing phosphorus and nitrogen. The ectomycorrhizae can be used as quality indicators for forest plants in nursery conditions since they help in their nutrition (Montoya, 2016).
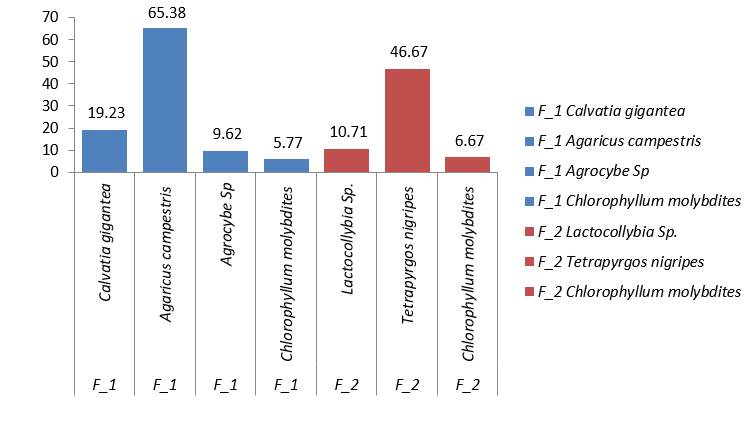
In Figure 7, it is shown that the species with the highest abundance in ecosystem one was Agaricus campestri, which was found near the depositions of grazing animals after a period of rainfall. Unlike ecosystem number two, Tetrapyrgos nigripes, which was associated with plant remains near the soil cultivated with Vigna unguiculata subspecies sesquipedalis after a period of rainfall (Figure 7).
The species Agaricus campestri develops in soils with high relative humidity and can be found in forests, gardens, trees, meadows (Barroetaveña et al., 2020). This species is of great importance to humans because it can be consumed at all stages of its life (Barroetaveña et al., 2020).
Coinciding with what was stated with Jagadish et al., 2019, which found the species Tetrapyrgos nigripes associated with plants of Anacardium occidentale in conditions of high relative humidity.
CONCLUSIONS
The species (Calvatia gigantea (Batsch) Lloyd, Agaricus campestris (L.), Agrocybe sp and ( Chlorophyllum molybdites (G. Mey.) Massee) are found in humid places with the presence of cattle, but this is not the case for Lactocollybia Sp, Tetrapyrgos nigripes (Fr.) E. Horak).
In the two ecosystems evaluated, the species with the highest abundance were Agaricus campestri and Tetrapyrgos nigripes.
REFERENCIAS BIBLIOGRÁFICAS
ÁLVAREZ-MANJARREZ, J., SOLÍS RODRÍGUEZ, A.U., VILLARRUEL-ORDAZ, J.L., ORTEGA-LARROCEA, M. del P., GARIBAY-ORIJEL, R., ÁLVAREZ-MANJARREZ, J., SOLÍS RODRÍGUEZ, A.U., VILLARRUEL-ORDAZ, J.L., ORTEGA-LARROCEA, M. del P. y GARIBAY-ORIJEL, R., 2021. Micorrizas del bosque tropical caducifolio y otras simbiosis fúngicas. Acta botánica mexicana [en línea], no. 128. [Consulta: 14/11/2022]. ISSN 0187-7151. DOI 10.21829/abm128.2021.1906. Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S0187-71512021000100149&lng=es&nrm=iso&tlng=es . [ Links ]
AZA, P., MOLPECERES, G., RUIZ-DUEÑAS, F.J. y CAMARERO, S., 2021. Expresión heteróloga, ingeniería y caracterización de una nueva lacasa de Agrocybe pediades con propiedades prometedoras como biocatalizador. En: Accepted: 2022-04-13T08:22:06Z, Sociedad Española de Microbiología [en línea], Disponible en: https://digital.csic.es/handle/10261/266943. [ Links ]
BARROETAVEÑA, C. y TOLEDO, C.V., 2020. Diversity and Ecology of Edible Mushrooms from Patagonia Native Forests, Argentina. En: PÉREZ, J. et al (eds.), Mushrooms, Humans and Nature in a Changing World: Perspectives from Ecological, Agricultural and Social Sciences [en línea]. Cham: Springer International Publishing, pp. 297-318. [Consulta: 14/11/2022]. ISBN 978-3-030-37378-8. Disponible en: Disponible en: https://doi.org/10.1007/978-3-030-37378-8_11 . [ Links ]
BECERRA, J. y MATEOS, A., 2020. Chlorophyllum molybdites, una especie foránea, recolectada en un parque de Mérida, primera cita en Extremadura. Bol. Inf. Soc. Micol. Extremeña [en línea], vol. 19, no. 18. Disponible en: https://www.researchgate.net/publication/347590392_Chlorophyllum_molybdites_una_especie_foranea_recolectada_en_un_parque_de_Merida_primera_cita_en_Extremadura. [ Links ]
BELTRÁN DELGADO, Y., MORRIS QUEVEDO, H., DOMÍNGUEZ, O.D., BATISTA CORBAL, P. y LLAURADÓ MAURY, G., 2021. COMPOSICIÓN MICOQUÍMICA Y ACTIVIDAD ANTIOXIDANTE DE LA SETA Pleurotos ostreatus EN DIFERENTES ESTADOS DE CRECIMIENTO. Acta Biológica Colombiana [en línea], vol. 26, no. 1, pp. 89-98. [Consulta: 14/11/2022 ]. ISSN 0120-548X. DOI 10.15446/abc.v26n1.84519. Disponible en: Disponible en: http://www.scielo.org.co/scielo.php?script=sci_abstract&pid=S0120-548X2021000100089&lng=en&nrm=iso&tlng=es . [ Links ]
CIFUENTES, J., VILLEGAS, M.. y PÉREZ, R. L., 1986. Hongos. (comp). En: LOT, A. y CHIANG, F. Manual de herbarios. S.l.: Consejo Nacional de Flora Mexico, D.F., [ Links ]
DESSING, H., ECKBLAD, F.E. y LANGE., M., 2000. Pezizales. Bessey. Revista Nordic Macromycetes, vol. 1, pp. 309. [ Links ]
DUEÑAS, Y.D., BAÑOS, Y.S. y GUANCHE, L.H., 2021. Efecto de los bioestimulantes sobre la germinación y crecimiento de plántulas de Vigna Unguiculata Subsp. Sesquipedalis l. Cv. Cantón 1. Revista Científica Agroecosistemas [en línea], vol. 9, no. 1, pp. 11-17. [Consulta: 26/01/2023]. ISSN 2415-2862. Disponible en: Disponible en: https://aes.ucf.edu.cu/index.php/aes/article/view/442 . [ Links ]
GALINDO-FLORES, G., CASTILLO-GUEVARA, C., CAMPOS-LÓPEZ, A. y LARA, C., 2015. Caracterización de las ectomicorrizas formadas por Laccaria trichodermophora y Suillus tomentosus en Pinus montezumae. Botanical Sciences [en línea], vol. 93, no. 4, pp. 855-863. [Consulta: 14/11/2022]. ISSN 2007-4298. DOI 10.17129/botsci.200. Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S2007-42982015000400016&lng=es&nrm=iso&tlng=es . [ Links ]
GARCÍA, J., PEDRAZA, D., SILVA, C.I., ANDREADE, R.L. y CASTILLO, J., 1998. Hongos del estado de Querétaro. S.l.: Hear Taller Gráfico, Querétaro. [ Links ]
HERNÁNDEZ, A., PÉREZ, J.M., BOSCH, D. y CASTRO, L., 2015. Clasificación de los Suelos de Cuba. Mayabeque, Cuba: Ediciones INCA. [ Links ]
HOSEN, I. et al. 2016. Lactocollybia subvariicystis, a new species of little known genus Lactocollybia from subtropical south China. Mycosphere [en línea], vol. 7, pp. 794-800. DOI 10.5943/mycosphere/7/6/10. Disponible en: https://www.researchgate.net/publication/310674411_Lactocollybia_subvariicystis_a_new_species_of_little_known_genus_Lactocollybia_from_subtropical_south_China. [ Links ]
JAGADISH, B.R., SRIDHAR, K. y H R, D., 2019. Macrofungal assemblage with two tree species in scrub jungles of south-west India. Studies in Fungi [en línea], vol. 4, no. 1, pp. 79-89. DOI 10.5943/sif/4/1/10. Disponible en: https://www.researchgate.net/publication/333234803_Macrofungal_assemblage_with_two_tree_species_in_scrub_jungles_of_south-west_India. [ Links ]
JIMÉNEZ NIEVA, F.J., SÁNCHEZ GONZÁLEZ, F.D.A. y CAETANO SÁNCHEZ, C., 2022. «Hongos. Ecología y biodiversidad en ecosistemas litorales de Huelva». Biología de Huelva: naturaleza, biodiversidad, bioindicadores y biomarcadores [en línea]. S.l.: Universidad de Huelva, pp. 145-186. ISBN 978-84-18984-95-2. Disponible en: http://rabida.uhu.es/dspace/bitstream/handle/10272/21312/Vegetacion_general_de_Huelva.pdf?sequence=2. [ Links ]
LÓPEZ, A. y GARCIA, J., 2005. Tetrapyrgos nigripes Fungi: Agaricales:Tricholomataceae. Funga Veracruzana [en línea], vol. 86, pp. 1-4. Disponible en: https://www.researchgate.net/publication/266675621_Tetrapyrgos_nigripes_Fungi_AgaricalesTricholomataceae. [ Links ]
MARTÍNEZ NEVÁREZ, L.E., SARMIENTO LÓPEZ, H., SIGALA RODRÍGUEZ, J.Á., ROSALES MATA, S. y MONTOYA AYÓN, J.B., 2016. Respuesta a la inoculación inducida de Russula delica Fr. en plantas de Pinus engelmannii Carr. en vivero. Revista mexicana de ciencias forestales [en línea], vol. 7, no. 33, pp. 108-117. [Consulta: 14/11/2022]. ISSN 2007-1132. Disponible en: Disponible en: http://www.scielo.org.mx/scielo.php?script=sci_abstract&pid=S2007-11322016000100108&lng=es&nrm=iso&tlng=es . [ Links ]
SALCIDO-RUIZ, S., PRIETO-RUÍZ, J.Á., GARCÍA-RODRÍGUEZ, J.L., SANTANA-AISPURO, E. y CHÁVEZ-SIMENTAL, J.A., 2020. Mycorrhiza and fertilization: effect on the production of Pinus engelmannii Carr. in nursery. 2020. Revista Chapingo serie ciencias forestales y del ambiente [en línea], vol. 26, no. 3, pp. 327-342. Disponible en: https://www.semanticscholar.org/paper/Mycorrhiza-and-fertilization%3A-effect-on-the-of-in-Salcido-Ruiz-Prieto-Ru%C3%ADz/88a5ef4116a935d1040a177b02e464a9156625d7. [ Links ]
VERMA, R.K., MISHRA, S.N., PANDRO, V. y THAKUR, A.K., 2018. Diversity and Distribution of Calvatia Species in India: A New Record from Central India. International Journal of Current Microbiology and Applied Sciences [en línea], vol. 7, no. 09, pp. 2540-2551. [Consulta: 14/11/2022]. ISSN 2319-7692, 23197706. DOI 10.20546/ijcmas.2018.709.316. Disponible en: Disponible en: https://www.ijcmas.com/abstractview.php?ID=9951&vol=7-9-2018&SNo=316 . [ Links ]
Received: October 24, 2022; Accepted: March 25, 2023











 texto em
texto em