Mi SciELO
Servicios Personalizados
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO
Links relacionados
-
 Similares en
SciELO
Similares en
SciELO
Compartir
Revista Cubana de Farmacia
versión On-line ISSN 1561-2988
Rev Cubana Farm v.45 n.1 Ciudad de la Habana ene.-mar. 2011
PRODUCTOS NATURALES
Climate influence on chemical composition and antioxidant activity of Justicia pectoralis Jacq.
Influencia del clima en la composición química y la actividad antioxidante de Justicia pectorales Jacq
Ph.D. Jonh Jairo Méndez ArteagaI; M.Sc. Elizabeth Murillo PereaII; c Ph.D. Edinson Yara VarónIII; M.Sc. Wilson Fabián Suescún OspinaII; Ph.D. Jaime Niño OsorioIV; c Ph.D. Oscar Marino Mosquera MartínezV
IDepartament of Chemistry. University of Tolima. Ibagué-Tolima, Colombia.
IIDepartament of Chemistry. University of Tolima. Ibagué-Tolima, Colombia.
IIIDepartament of Chemistry. University of Tolima. Ibagué-Tolima, Colombia.
IVSchool of Technological Chemistry-University Thecnological of Pereira. Pereira-Risaralda, Colombia.
VSchool of Technological Chemistry-University Thecnological of Pereira. Pereira-Risaralda, Colombia.
ABSTRACT
In this paper, the influence of the altitude in the phenols and flavonoids contents and the antioxidant activity of the extracts of different polarities of Justicia pectoralis Jacq(Acanthaceae) was evaluated. There was found that the plants cultivated under the Ibagué-Tolima's climate and soil conditions, can be a source of antioxidant compounds, especially in water preparations. The chromatographic analysis revealed that J. pectoralis extracts have mainly flavonoids of the flavonone type, as apigenine. A greater (or The greatest) content of flavonoids was detected in the ethanolic extract of the plant samples grown at 1 265 m.a.s.l. (meters above sea level) (2 748,03 mg/L). The altitude level does not seem to have any influence on the functional properties, neither on the phytophenols content. The J. pectoralis Jacq can be considered one species with a high therapeutic potential and with good commercial opportunities.
Key words: Antioxidant activity, Justicia pectoralis (Acanthaceae), phenolic content, Flavonoids.
RESUMEN
En este trabajo se evaluó la influencia de la altitud en el contenido de fenoles y flavonoides y la actividad antioxidante de los extractos de diferentes polaridades provenientes de Justicia pectorales (Acanthaceae). Se halló que las plantas cultivadas en las condiciones del suelo y el clima de la zona Ibagué-Tolima pueden constituirse en compuestos antioxidantes, especialmente en preparados acuosos. El análisis cromatográfico reveló que los extractos de J. pectorales poseen en lo fundamental flavonoides del tipo flavona como es la apigenina. Un mayor contenido (sino el mayor) de flavonoides se detectó en el extracto etanol obtenido de las muestras de la planta cultivadas a 1 265 metros por encima del nivel del mar (2 748,03 mg/L). La altitud no parece influir en las propiedades funcionales, ni en el contenido de fitofenoles del extracto. La J. pectorales puede considerarse una especie de gran potencial terapéutico y buenas posibilidades de comercialización.
Palabras clave: actividad antioxidante, Justicia pectorales (Acanthaceae), contenido de fenoles, flavonoides.
INTRODUCTION
Day after day, the number of diseases in which the etiology connected to oxidative stress, produced when reactive oxygen species (ROS) or the nitrogen (NOS); known as nitrogen stress. These diseases over number, in an independent or a joint way, the antioxidant defenses on our organism.1 The nervous tissues seem to be the best place for those pro-oxidant compounds since they have chemical characteristics such as high quantities in polyunsaturated fatty acids, high concentrations of iron and low quantity of antioxidant enzymes.
Some medicinal plants and cooking herbs turn out to be of a particular interest due to the fact that they can be used for the production of preparations that possess compounds with a significant antioxidant effect. This effect is said to have happened by the phenolic type, such as flavonoids.2 Phenolic acids and diterpenesphenolics.3 The antioxidant activity of the phytophenols is due to its redox property, which plays an important role in capturing the free radicals, inhibiting the singlete and triple oxygen, nitrogen oxide or decomposition of peroxides.4
The phytophenols have usually been considered as anti nutritious factors due to their abilities to chelate metals, their adverse effects to precipitate proteins, since most of them are present as glycosides which lead lyses in the intestine. Consequently, this could imply in the blocking of heterocyclic aromatics amines that promote the gastric and colon carcinogenesis; despite the given fact, literature directly correlates the antioxidant activity.5-7 Specifically, the flavonoids are the most important group of natural phenols and more than 4 000 have been identified on severalsuperior and inferior species of plants.
Justicia pectoralis Jacq. (Acanthaceae) is known as «Bull tamer». This plant is widely used by the Tolima regions ethnics and it represents the publicly called «traditional medicine», which has been transmitted from generation to generation due to its ethno medicine relevant properties. This has helped increase the interest of researchers in studying the biological, chemical and pharmacological properties of the species.8-11
In the present work, a group of tests was carried out in orderto knowthe correlation of the antioxidant activity with the phenolic and flavonoids total contents of J. pectoralis. In this line an aqueous and organic extracts of the plant were analyzed. This plant was grown in two thermal floors with the aim of knowing the altitude effect in its chemical andbiological properties and to contribute in the knowledge of our native flora.
METHODS
Chemicals
All the chemicals used were of analytical reagent from Merck or HPLC grade Sigma Chemical Co (St. Louis,Missouri, USA).
Plant Material
The aerial parts of J. pectoralis were collected in Ibagué (4° 26' N y 75° 13' O, 24 ± 1 ºC. Colombia), at 1 265 and at 1 030 m.a.s.l., under the conditions of a semitechnical cultivation. A sample was the carried to the Toli Herbarium at University of Tolima; it was referenced with the code 008989. The collected plant material was air-dried under the shade and ground in a manual mill and kept at room temperature (27 ± 2 °C, 48 h) in order to protect it from the direct light.
Preparation of the plant extracts
A sample of ground anddried material was soaked in ethanol and ethyl acetate (48 h, rate 1:20, plant/solvent). The solvent was renewed every 24 h until the sample was run out. Additionally, some water extract was used (rate 1:20, plant/solvent; 30 min) by the decoction method according to the methodology used by the ethnic groups. The organic and aqueous extracts were concentrated under vacuum at 40 ºC to obtain a viscous material. This material was kept (4 ºC) in labeled glasses.
Determination of the phenolic content
The content of total phenolic compounds of the extracts was quantified by using the Folin-Ciocalteu (FC) reactive, according to the procedure by Singleton and Rossi.12 The extract (1 mL, 40 µg/mL) was measuredwith water up to 50 mL; 1 mL of this solution was mixed with the FC (2.5 mL, diluted1:1 on distilled water) reactive and sodium carbonate (2 mL 7.5 %); the mixture was incubated (10 min, 50 oC). This mixture was cooled and the reaction mixture absorbance was measured at 760 nm. The blank was prepared with all the reagents except the sample. The gallic acid was chosen as a standard using 6 point standard curve (10-50 µg/mL). All the tests were conducted in triplicate. The total phenolic content was expressed as milligram gallic acid/g extract.
ANTIOXIDANT ACTIVITY
Free radical scavenging activity
The interaction of constituents of samples was determined based on their ability to react with the stable 2,2-diphenyl-1-picryl-hydrazyl (DPPH) free radical. The procedure described by Ohinishiwas not widely adopted.13 The assay was carried out with aliquots of 1mL of each sample added to 3 mL of an ethanol solution of DPPH (0.1 mM), the mixture was incubated at a temperature of 25 °C for 30 min, and the absorbance of each solution was determined at 517 nm.
The scavenging effect of the radical of each sample was calculated and compared with the scavenging effect of 2.6 di-butyl-4-methylphenol (BHT), in the concentrations of 10-100 µg/mL All the tests were conducted in triplicate. The scavenging effect of the DPPH radical was calculated using the following equation:
![]()
Ferric Reducing Antioxidant Power
The ferric reducing antioxidant power (FRAP) assay suggests an indication of the reducing ability of the plant extract. The reducing power of J. pectoralisethanolic, ethyl acetate and aqueous extracts was determined using the method described previously by Choi y Hwang.14 A serial dilution of the extract was performed (40-160 µg/mL) in (2.5 mL, 0.2 M, pH, 6.6) phosphate buffer containing 1 % ferrocyanate (2.5 mL). The mixture was incubated (50 ºC, 20 min). 10 % trichloroacetic acid (TCA, 2.5 mL) was added to a portion of this mixture (6 mL) and centrifuged (3 000 g /10 min). The supernatant was separated and mixed with distilled water (2.5 mL) containing 1 % ferric chloride (0.5 mL). The absorbance of this mixture was measured (700 nm). The increase of the variable permitted to know the reducing power. The intensity in absorbance could be the measurement of antioxidant activity of the extracts and compared with the activity of Ascorbic acid (AA) and Gallic acid (AG), at 15 µg/mL, used as reference standards. All the tests were conducted in triplicate.
Determination of Fe2+-chelating ability
The chelation of ferrous ion by the extracts, gallic acid, BHT and EDTA, used as standard, was measured using the method described by Diniset al.15 The J. pectoralis extracts (320 µg/mL), was mixed with FeCl2 (0.1 mL, 2.0 mM) and after 30 min, 3-(2-pyridyl)-5,6-bis(4-phenylsulfonic acid)-1,2,4-triazine (ferrozine 0.2 mL, 5 mM) was added. The resulting mixture was shaken vigorously and left to stand for 10 min at room temperature. The absorbance of the resulting solution was measured (562 nm). The lower the absorbance of the reaction mixture, the higher the Fe2+-chelating ability. The percentage of inhibition of ferrozine-Fe2+ complex formation was calculated from the absorbance ratio to that of the blank without any sample and calculated using the following equation:
![]()
A0 indicates the absorbance of the control and A1 the absorbance in the presence of the J. pectoralis extracts, artificial antioxidants or EDTA.
Scavenging of hydrogen peroxide
The ability of the extracts to scavenge hydrogen peroxide was determined according to the method of Ruchet al.16 A solution of hydrogen peroxide (40 mM) was prepared in phosphate buffer (pH 7.4). The solution concentration was measured by using a spectrophotometer at a wave length of 230 nm by using a constant molar absorbing of 81 n-1 L cm-1 in distilled water. The percentage of hydrogen peroxide scavenging by the extract and standard compounds was calculated as follows:
![]()
AB was the absorbance of the control and AM was the absorbance in the presence of the sample of extract and standard. The activity of extracts was contrasted with the ascorbic acid, gallic acid and rutin (15 µg/mL).
Qualitative analysis of flavonoides
Thin-layer chromatography (TLC) was performed on precoated 20 x 20 cm TLC plates coated with 0.25 mm layers of silica gel 60 F254 (Merck). After application of the extract and standard solutions (10 µL), the plates were developed on 19 cm in paper-lined all- glass chambers (Desaga, Germany) previously left to equilibrate for at least 30 min. Two chromatography solvents were used: ethyl acetate/formic acid/acetic acid/water, 100:11:11:26 (V/V) and ethyl n-hexane /acetate/formic acid, 62:28:10 (V/V) (Visualization of the flavonoids was achieved by spraying the sheets with AlCl3 al 1 %.17 Typical intense fluorescence in UV light at l= 365 nm was produced immediately on spraying (flavonoids appeared as orange-yellow bands).
HPLC analysis of flavonoids
The HPLC system was equipped with a Hewlett Packard 1 100 serial system, consisting of a quaternary pump, online degasser, auto-sampler, column heater and variable wavelength detector. Separation was achieved on a reversed phase column (Hypersil ODS 5 µm, 125 x 4 mm), provided with a C18 guard column and methanol-water (7:3), v/v, isocratically), was employed as the mobile phase. The flow rate was kept constant at 0.7 mL/min and the peaks were identified using Diode array detector (DAD) at 335 nm. The temperature of the column during analysis was maintained at 40 ºC. The injection volume was 20 µL each time.
Calibration
Stock solutions of apigenine (10-400 µg/mL) were prepared as well as 2.0 mL of the stock solution. Each was put into a 25 mL volumetric flask and adjusted with methanol for the standard curves. The analysis of the lineal regression, the correlation coefficient (r2), the relative standard deviation (%RSD), or variation coefficient (VC) was determined using the Software of (Chemstation LC unit). The identification of the flavonoids was made taking into account the comparison of the retention time (tR), in the solutions of the different extracts of J. pectoralis and the patterns naringenine, Hesperidin, Myricetin, Shikimic Acid and choloregenic acid.
Statistical analysis
Experimental results are expressed as Means ± SD. All measurements were replicated three times. The analysis on lineal regression was made to calculate the dose-response relationship of the standard solutions and also for the samples of the analyzed extracts. The correlation degree between the variables was expressed through the correlation coefficient rxy. In order to determine if there was an effect of the altitude in regards to the antioxidant activity, an ANOVA was used. The differences between the treatments were tested by using the Post-hoc (LSD, Tukey and Bonferroni) application. The STATISTICS 7.0 software program was used.
RESULTS
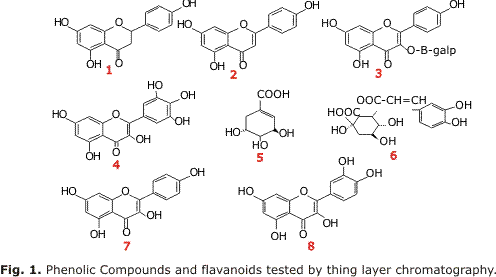
The types of flavonoids in the extracts of J. pectoralis were qualified by thin-layer chromatography based on the following patterns: (1) Naringenin, (2)Apigenine, (3) Hesperidin, (4). Myricetin, (5) Shikimic acid, (6) Chrologenenic acid, (7) Kaempferol and (8) Quercetin (fig. 1). The thin layer chromatography analysis revealed that the samples had mainly flavonoids, especially of the flavone kind, just like the apigenine, particularly on the ethanol extracts (1) and the ethyl acetate (5) obtained from the collected plant piece at 1 265 m.a.s.l., respectively.
Table 1 shown the performance and the content of total phenols for every one of the extracts of J. pectoralis, calculated by the regression equation of the calibrating curve: y= 0.0167x - 0.0534, r2= 0.9996 and are expressed as equivalent milligrams of Gallic acids per extracted gram (EAG/gEx). As seen, the ethanol was able to get the greatest quantity of phytophenols. The highest altitude level seemed to promote the biosynthesis of these metabolites.
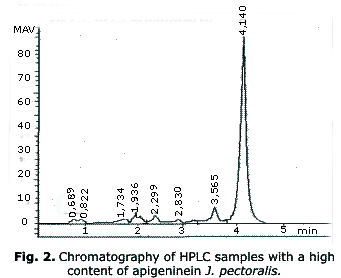
Over the base of the qualitative chromatographic results on thin layer, the apigenine was used as an external pattern to quantify the flavonoids present on in the J. pectoralis extracts. Under the chromatographic conditions of HPLC, the apigenine showed a retention time of (tr), 4.25 min average and a calibration curve of (r2= 0.9984). The mobile phase on the analysis showed an adequate separation degree, the resolution of the peaks was appropriate and there was a low noise; this permitted to obtain a stable base line during the several analyses (6 min); these parameters were done in all the trials.
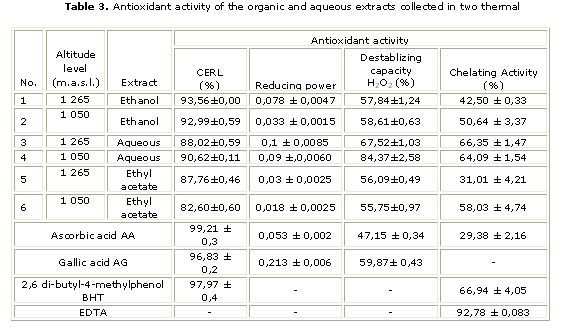
On table 2 the content of flavonoids from all the samples of J. pectoralis is shown, while on figure 2 the chromatogram of the sample with the greatest content of flavonoids, is shown influence on the flavonoids content. On the other hand, the table 3 shows the antioxidant potential of J. pectoralis.
DISCUSSION
The presented values for the extracts 1 and 5 suggest that the plant has structural diversity on flavonoids; being the polar ones the greatest, either hydroxide and/or glycosides. In general, all the extracts showed a big capacity to react with the pro-oxidant species, which is beneficial for the human health since the contents of phytophenols in the ethanol and aqueous extracts showed a greater stabilizing capacity of the free radical (CERL) than the corresponding ones of the ethyl acetate.
The capacity to interact with free radical of the phytophenols is considered to be dependent on the location of the functional groups on its structure; so the number and configuration of the donating hydroxiles of H+ are of a fundamental importance to act as antioxidants. Moreover, the biological properties (vasodilators, anticancer, antibacterial, antiviral), pharmacological ones (anti-inflammatory and anti-allergic) and biochemical (estrogenic effects, inhibitors of the phosphatase Enzymes A2, Cyclooxygenase, lipoxygenase, glutathionereductase y xanthine oxidase) of the flavonoids have been widely reported.18-20 To this wide spectrum there is added the skill of these phytocompounds to stabilize radical free,21,22 sustained in the high number of hydroxile phenolic groups joined the structural rings that typically they possess.
The antioxidant potential of the extracts was re-confirmed by determining its capacity to reduce the ion Fe+2 a Fe+3, which is supported in Fenton reaction. This is traditionally defined as the catalytic generation of the radical •OHgiven the chain reaction between the iron ion and hydrogen peroxide.
It is important to mention that the reducing capacity of a product can be determined by its ability to bind metallic ions that act as catalyzers in reactions that generate free radicals; for instance the Fe+2 or the Cu+ or the stabilizing capacity of free radicals, already created or the inhibitory potential in beginning reactions of these chemical species, or to its potential to decompose peroxides.23
Even though most extracts showed results higher than 50%, none was comparable to EDTA, a perfect chelating agent. It has been reported that the chelating agents form sigma binds with the metal, and then, it is grouped as secondary antioxidants, due to fact that they can diminish the redox potential, generating the oxidized form of the metallic ion.24
It is important to mention that the H2O2 generates hydroxyls in a catalyzed reaction by metallic ions (Fe+2 o Cu+); the hydroxyl radical is a highly reactive species that attacks almost all bio-molecules. Then, removing the hydrogen peroxide is an important measurement of the antioxidant activity of the plant extracts.
Given the fact that the action mechanisms of the antioxidants are diverse and depend on intrinsic factors (structure, solubility, among others) and extrinsic (nature of the pro-oxidizing unit, reducing factor, and sort of trial).25 It is justifiable to use a set of in vitro trials with the aim to estimate the antioxidant action of a plant product; pure compound, extract or essential oil.
In this study several solvents were in use for extracting the antioxidant ones, the ANOVA applied to itself to the solvent variable extractor sheltered inside the height (F4.47= 1.599, p= 0,1900 y F1.47= 1.1698, p= 0.2849, respectively). The obtained statisticians indicated that influence of the height does not exist in the behavior of the constituent ones of the extracts.
Nonetheless, when applying ANOVA to the solvents in an isolated way, it was revealed a significant difference between them, and the marker F2.50= 3.2398, p= 0.0475 marker could be gotten.
In order to determine the difference between them, several posterior tests were carried out (post hoc): the LSD test, the Bonferroni test and the Tukey test (MS= 313.64, df= 50.00), which allowed to know that there were significant differences between the antioxidant activity of the secondary metabolites of the extracted J. pectoralis with water and those of ethyl acetate, but not between the latter and the ethanol.
The shown responses by the J. pectoralis extracts in the four tests with the antioxidant activity were higher in the aqueous extracts, which could be caused by the greatest content of polarity compounds, as phenols and flavonoids, usually known for being great antioxidant agents.
This study allows to infer the bioactive constituents of J. pectoralis could be included in the non enzymatic secondary antioxidant group among those ones are Vitamin C, since they come from the formation of new free radicals making them in less inconvenient molecules before reacting or can avoid the formation of free radicals from other molecules. Besides it is clear the capacity to capture free radicals and then, it leads to stopping its chain reaction.26-28
It is of a particular interest to see that in the related literature on this study does not show similar researches, where the total phenolic and flavonoids of J. pectoralis content is evaluated and, additionally the antioxidant activity on different polarity extracts is tested by relating it with the altitude level where the plant grows.
CONCLUSIONS
The work showed that the Justicia pectoralis, cultivated under the conditions of climate and soil in Ibagué-Tolima, can be sources of antioxidant compounds given the extracts of different polarity. The height where the plant is cultivated does not seem to influence on its functional properties, as well as the content of phytophenols (phenols and flavonoids).
The sort of used solvent to extract the active constituents of the plant influence in the antioxidant activity shows, being the aqueous extract obtained by decoction from the greatest potential, which scientifically supports the popular local knowledge in Tolima.
The shown antioxidant action by J. pectoralis allows inferring that it can be a plant with a high therapeutic potential and commercial use. The total content of the phenols and flavonoids show that the extracts of J. pectoralis are possible to have a good antibacterial and anti fungi activity, which is being subjected to evaluation.
ACKNOWLEDGMENTS
To the Department of Chemistry, to the Office of Investigations and Scientific Development of the University of the Tolima for the economic and logistic support granted to this investigation.
REFERENCES
1. Mavi A, Terzi Z, Ozoen U, Yildirim A, Coskun M. Antioxidantproperties of some medicinal plants: Prangosferulacea(Apiaceae), Sedumsempervivoides(Crassulaceae), Malvaneglecta(Malvaceae), Cruciatataurica(Rubiaceae), Rosa pimpinellifolia (Rosaceae), Galium verum subsp. Verum (Rubiaceae), Urtica dioica (Urticaceae). Biol Pharm Bull. 2003;27:702-5.
2. Perez TG. Los flavonoides: antioxidantes o prooxidantes. Rev Cubana Invest Biomed. 2003;22:48-57.
3. Javanmardi J, Stushnoft C, Vivanco J. M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chemistry. 2003;83: 547-50.
4. Osawa T. Novel natural antioxidant for utilization in food and biological systems. Posharvest systemsPosharvest biochemistry of plant food-materials in the tropicals. Tokyo: Japan Scientific Societies Press; 1994. p. 241-51.
5. Miliauskas G, Venskutonis PR, Van Beek T. A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chemistry. 2003;41:753-8.
6. Deliorman O. Novel flavonone glucoside with free radical scavenging properties from Galiumfissurense. Pharm Biol. 2003;41:475-8.
7. Faure M, Lissi E, Torres RY, Videla LA. Antioxidant activities of lignans and flavonoids. Phytochemistry. 1990;29:3773-5.
8. Rodríguez FCA, Hechevarría SI, Fuentes FVR. Fecha y distancia de plantación en el cultivo del tilo (Justicia pectoralis Jacq. var. stenophylla Leonard). Rev Cubana Plantas Med 2003; 1. Disponible en:
http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962003000100004&lng=es
9. Sanchez GE, Fuentes HL, Chávez FD, Rodriguez FC. Estudio farmacognóstico de Justicia pectoralis Jacq. var. stenophylla Leonard. Rev Cubana Plantas Med 2003;8(3). Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1028-47962003000300005&lng=es
10. Montero R, Pérez AG, Fernández EN, Bada B, Arteaga PM, Mancebo RA. Estudio genotóxico in vivo de 6 extractos de plantas medicinales en células de la médula ósea de roedores. Rev Toxicol. 2001;18:75-8.
11. Perez TG, Rivero MR, Pardo RZ, Rodriguez CJ. Evaluación de la actividad antioxidante de Justicia pectoralis Jacq. Revista Cubana Invest Biomed. 2001; 20: 30-33. Disponible en: http://scielo.sld.cu/scielo.phpscript=sci_arttext&pid=S0864-03002001000100006&lng=es
12. Singleton V, Rossi JA. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am J Enol Vitic. 1965; 16:144-58.
13. Ohinishi M. Inhibitory effects of chlorogenic acids on linoleic acid peroxidation and haemolysis. In: Miceli N. Anti-inflammatory activity of extract and fractions from Nepetasibthorpii Bentham. J Ethnopharmacol. 2005;97:261-6.
14. Choi EM, Hwang JK. Screening of Indonesian medicinal plants for inhibitor activity on nitric oxide production of RAW264.7 cells and antioxidant activity. Fitoterapia. 2005;76:194-203.
15. Dinis TCP, Madeira VMC, Almeida LM. Action of phenolic derivates (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch Biochem Biophys. 1994;315:161-9.
16. Ruch RJ, Cheng SJ, Klauning JE. Prevention of cytotoxixity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003-8.
17. Wagner H, Bladt S. Plant drug analysis a thin layer chromatography atlas. Berlin: Springer-Verlag; 1996. p. 384.
18. Cody V, Middleton E, Harborne JB, Beretz A. Plant flavonoids in biology and medicine-Biochemical, pharmacological and structure-activity relationships. New York: Marcel Dekker; 1996. p. 281-96.
19. Cody V, Koehrle J, Auf'mkolk M, Hesch RD. Structure-activity relationships of flavonoid deiodinase inhibitors and enzyme active-site models. In: Cody V, Middleton E, Harborne Jr. JB. Plant Flavonoids in Biology and Medicine. Biochemical, Pharmacological and Structure-activity Relationships, Liss. New York: Marcel Dekker; 1986. p. 373-82.
20. Das NP. Flavonoids in biology and medicine III: current issues in flavonoid research. Singapore: National University of Singapore Press; 1990. p. 227-35.
21. Gonçalves C, Dinis T, Batista MT. Antioxidant properties of proanthocyanidins of Uncariatomentosa bark decoction: a mechanism for anti-inflammatory activity. Phytochemistry.. 2005;66:89-98.
22. Jasril, Mooi LY, Lajis NH, Ali AM, Sukari MA, Rahman AA, Toman AG, Kikuzaki H, Nakatani N. Antioxidant and antitumor promoting activities of the flavonoids from Hedychiumthyrsiforme.Pharma Biol. 2003;41:506-11.
23. Nordberg A. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287-312.
24. Gordon MH. The mechanism of antioxidant action in vitro. In: Hudson BJF, ed. Food Antioxidants. Elsevier: Applied Science; 1990. p. 1-18.
25. Mathew S, Abraham E. In vitro antioxidant activity and scavenging effects of Cinnamomumverum leaf extract assayedby different methodologies. Food Chemical Toxicol. 2006;44:198-206.
26. Chae S, Kim JS, Kang KA, Bu HD, Lee Y, Hyun JW, Kang SS. Antioxidant activity of Jionoside D from Clerodendrontrichotomun. Biol Pharm Bull. 2004;27:1504-8.
27. Céspedes T, Sánchez D. Algunos aspectos sobre el estrés oxidativo, el estado antioxidante y la terapia de suplementación. Rev Cubana Cardiol. 2000; 14:55-60. Disponible en: http://bvs.sld.cu/revistas/car/vol14_1_00/car08100.pdf
28. Ferrer D, Fonsec J, Cutiño I, Garcia R, Arce D. Radicales libres y su papel en la homeostasia neuronal. MEDISAN. 1999;3:5-11.
Recibido: 5 de octubre de 2010.
Aprobado: 13 de noviembre de 2010.
Ph.D. Jonh Jairo Méndez Arteaga. Departament of Chemistry -University of Tolima. Barrio Santa Helena, Ibagué-Tolima, Colombia. E-mail: jmendez@ut.edu.co