My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Cubana de Farmacia
Print version ISSN 0034-7515
Rev Cubana Farm vol.46 no.3 Ciudad de la Habana Jul.-Sept. 2012
ARTÍCULO ORIGINAL
Validation of a liquid chromatographic method for determination of sulphadoxine and pyrimethamine in whole blood spotted on filter paper
Validación de un método de cromatografía líquida para determinar la presencia de sulfadoxina y pirimetamina en muestra de sangre secada sobre papel de filtro
Dra. Cs. Diana Margarita Márquez Fernández,I Dra. Cs. Adriana Lucía Pabón Vidal,II MSc. Carlos Alberto López Córdoba,III MSc. Silvia Blair TrujilloII
I Facultad de Química Farmacéutica. Universidad de Antioquia. Medellín, Colombia.
II Facultad de Medicina. Sede de Investigación Universitaria (SIU). Universidad de Antioquia. Medellín, Colombia.
III Instituto de Química. Facultad de Ciencias Exactas y Naturales. Universidad de Antioquia. Medellín, Colombia.
ABSTRACT
Objective: to validate an analytical method for simultaneous determination and quantification of sulphadoxine and pyrimethamine in human blood dried onto filter paper, whose cost and analysis time can be reduced.
Methods: whole blood spotted on filter paper of a healthy volunteer and solutions of sulphadoxine-pyrimethamine standard mixture were used. HPLC separations were carried out on Agilent equipment using a LiChrospher® column C18 with a mobile phase acetonitrile/0.1 M potassium phosphate buffer at pH 3.0 (1:1) for eight minutes under isocratic conditions. A flow rate of 0.7 mL/min, and a 20 mL volume injection were used. External standard method for quantitation of analytes was used.
Results: the HPLC method described for the simultaneous determination of sulphadoxine and pyrimethamine in 100 mL of whole blood spotted on filter paper has been found to be linear, precise, accurate and selective. In this method, the sample preparation is simple using liquid-liquid extraction, and HPLC with ultraviolet detection is used.
Conclusions: a simple, fast and sensitive method for determination of sulphadoxine and pyrimethamine in human blood dried onto filter paper was validated. This method can be used for the monitoring of both metabolites in pharmacokinetic and clinical studies.
Key words: pyrimethamine, sulphadoxine, validation.
RESUMEN
Objetivo: validar un método de análisis para la determinación y cuantificación simultánea de sulfadoxina y pirimetamina en sangre humana secada sobre papel de filtro que sea rápido y barato.
Métodos: se usó sangre de un voluntario sano impregnada sobre papel de filtro y soluciones estándar de la mezcla sulfadoxina y pririmetamina. Las separaciones por cromatografía líquida de alta resolución (CLAR) se hicieron en un equipo Agilent sobre una columna C18 LiChrospher® con acetonitrilo/buffer fosfato de potasio 0,1 M a pH 3,0 como fase móvil, usando condiciones isocráticas durante 8 min. Se usó un flujo de 0,7 mL/min y un volumen de inyección de 20 mL. Para la cuantificación de los analitos se utilizó el método del estándar externo.
Resultados: el método CLAR descrito para la determinación simultánea de sulfadoxina y pirimetamina en 100 mL de sangre impregnada sobre el papel de filtro mostró linealidad, precisión, exactitud y selectividad. En este método la preparación de la muestra es simple ya que usa extracción líquido-líquido y detección ultravioleta.
Conclusión: se obtuvo un método validado que es simple, rápido y sensible para la determinación de sulfadoxina y pirimetamina en sangre humana impregnada sobre papel de filtro, que puede ser usado para el monitoreo de ambos metabolitos en estudios farmacocinéticos y clínicos.
Palabras clave: pirimetamina, sulfadoxina, validación.
INTRODUCTION
Combination of sulphadoxine and pyrimethamine has been used like an alternative therapy for the treatment of malaria in places in which Plasmodium falciparum is cloroquine-resistant. For determining the efficacy of both active ingredients, is a necessary take blood sample of infected people with the parasite and treated with these drugs. Owing to the risk of contagious with the human immunodeficiency virus (HIV) of laboratory personal is evident with the bad manipulation these samples, the risk has been minimized for impregnation of filter papers with blood samples and after dried.
Some studies related by the determination of sulphadoxine and pyrimethamine in plasma have been done,1-6 but only a study about determination of sulphadoxine and pyrimethamine from whole blood dried onto filter paper using solid phase extraction during the preparation of samples has been reported.7
Our intention is to make easier the analysis both analytes using a validated technique, which costs and analysis time can be decreased. In this technique, the sample preparation is simple using liquid-liquid extraction, and HPLC with ultraviolet detection is used.
METHODS
Reactives
Solvents employed for extraction were analysis grade: potassium phosphate monobasic (Merck), potassium phosphate dibasic (Carlo Erba), chloroform (Mallinckrodt), concentrated hydrochloric acid (J.T. Baker), and HPLC-grade solvents were utilized without further purification in HPLC separations (EM Science).
Equipment and chromatographic conditions
Micropipettes of 1-20 mL, 10-200 mL and 100-1 000 mL (Wilson), vortex mixer (Schott), centrifuge 5416 for eppendorf vials (Brikmann) and ultrasound (Ultrasonic LC60H Elma), were used. HPLC separations were carried out on Agilent equipment (isocratic pump, manual injector, programmable ultraviolet detector). The following chromatographic conditions were used: a LiChrospher® column C18 (150 × 10 mm, 5 m) with a precolumn RP-18 (Merck), using as mobile phase acetonitrile/0.1 M potassium phosphate buffer at pH 3.0 (monobasic potassium phosphate and dibasic potassium phosphate and) (1:1) under isocratic conditions for eight minutes. A flow rate, 0.7 mL/min and 20 mL volume injection, were used. Analytes were detected according follow conditions: 272 nm of 0 to 3 min for suphadoxine and 287 nm of 3 to 8 min for pyrimethamine. Retention times were 1.5 ± 0.2 and 4.3 ± 0.3 min for suphadoxine and pyrimethamine, respectively. External standard method for quantitation of analytes was used.
Reference standards
Reference standards of sulphadoxine base (Hoffman La Roche Inc.) and pyrimethamine base (Sigma Chemical Co/Wrair) were donated for El Centro Internacional de Entrenamiento e Investigaciones Médicas (CIDEIM-Cali, Colombia).
Preparation of samples
100 µL of blood of a healthy volunteer on a reference filter paper (Whatman # 3) were dried at room temperature and putted into a vial of 2 mL. The following solutions were added: 500 mL of sulphadoxine-pyrimethamine standard mixture at three levels of concentration (300 mg/mL, 200 mg/mL and 100 mg/mL for sulphadoxine, and 300 ng/mL, 200 ng/mL, and 100 ng/mL for pyrimethamine), 250 mL hydrochloric acid 0.1 M and 750 mL of acetonitrile. In brief, samples were centrifuged at 14 000 rpm by 15 min, after that organic phase was separated and 1 mL of potassium phosphate buffer at pH 5.6 was added and the sample was sonicated two minutes into an ultrasound. Immediately, 2.5 mL of chloroform were added and mixed on a vortex mixer. Sample was settled and chloroform phase was separated and evaporated. Dry residue was dissolved in 1 mL of mobile phase, filtered on a filter paper (0.45 mm) and injected into the liquid chromatograph.
VALIDATION PARAMETERS
Recovery studies
Standard mixture of sulphadoxine-pyrimethamine at three levels of concentration (50, 100 and 150 mg/mL and 50, 100 and 150 ng/mL of sulphadoxine and pyrimethamine, respectively, were added to blood samples of healthy individuals.
Selectivity
For determining the selectivity of method mobile phase, placebo (blood of a healthy volunteer without both analytes), sample added with both analytes and standard mixture were injected into the liquid chromatograph.8-15
Linearity and range
The method linearity was evaluated using a calibration curve with the following concentrations: 300, 200, 100, 50 and 10 mg/mL for sulphadoxine, and 300, 200, 100, 50 and 10 ng/mL for pyrimethamine. Calibration curves were constructed of peak-area measurement each analyte versus the concentration of corresponding standard solution.8-15
Precision
For determining method precision, a repeatability assay was done. 100 mg of sulphadoxine and 100 ng of pyrimethamine were added to sample that was injected ten times into the liquid chromatograph.8-15
Accuracy
Accuracy of the assay method was determined using nine samples that were analyzed at three levels of concentration and after the recovery percentages were calculated.8-15
Detection and quantification limits
The detection (LOD) and quantification (LOQ) limits were estimated with the zero concentration extrapolation method.15
Robustness
The robustness of the method was examined by making small, deliberate changes to conditions such as pH, mobile phase composition, and flow rate. pH effect was evaluated at pH selected ± 1.0 and standard solutions at 100 mg/mL and 100 ng/mL of sulphadoxine and pyrimethamine were used. The concentration of the mobile phase was varied at three levels (± 2 %) and flow rate was varied at three levels, 0.5, 0.7, and 0.9 mL/min.8-15
Stability of the analytes solutions
The stability of the analytes solutions were determined using sulphadoxine and pyrimethamine solution (100 mg/mL for sulphadoxine and 100 ng/mL for pyrimethamine) by keeping at ambient temperature for 48 h and analyzing.14,15
RESULTS
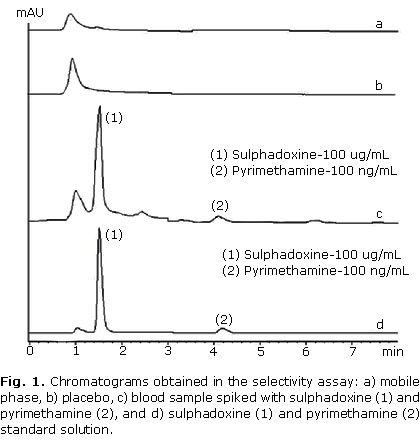
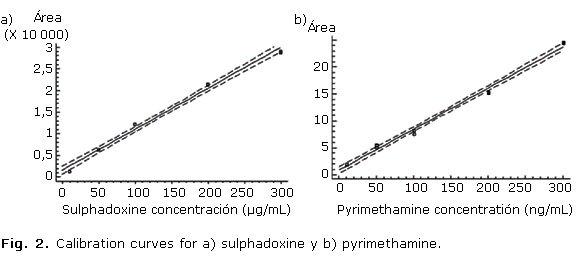
The recovery was studied at concentrations of 50 % (n= 4), 100 % (n= 7) and 150 % (n= 7) of the target level in the sample. The results are showed in the table 1. Figure 1 show the obtained chromatogram in assays done for determining method selectivity. Linearity was demonstrated at concentrations from 10 to 300 µg/mL for sulphadoxine and 10 to 300 ng/mL for pyrimethamine. Data from three replicated calibration curves were drawn (Fig. 2). The regression equation of sulphadoxine and pyrimethamine were Y= 715.508 + 97.7624 X and Y= 1.01052 + 0.0661872 X, respectively. Results of regression analysis are showed in the table 2.
The precision of the method was examined by performing intra-day by replicated (n= 10) injections of the mixed standard solution at medium concentration. The results for repeatability of injection of the solution at medium concentration showed a mean of 81.32 and 97.06 %, a standard deviation of 1.46 and 1.65, and a coefficient of variation of 1.80 and 1.70 % for sulphadoxine and pyrimetamine, respectively. Individual confidence intervals (X ± ts) were 78.07-84.57 % and 93.38-100.74 %, and mean confidence intervals (X ± ts/Ön) were 78.07-84.57 % and 95.90-98.22 %, for sulphadoxine and pyrimetamine, respectively, at a 95 % of confidence level for n-1 degrees of freedom with ttables= 2.228.
The accuracy of the method was confirmed by measurement of recovery by the standard addition method. Three different quantities (low, medium, and high) of the authentic standards were added to blood samples. The mixtures were extracted by the method described previously and analyzed by use of the HPLC. The quantity of each component was subsequently obtained by use of the corresponding calibration plots and each set of additions was repeated three times. Results of accuracy test of the method are showed in the table 3.
The values obtained of LODs were 30.50 ng/mL and 1.39 ng/mL, and LOQs were 45.78 ng/mL and 1.49 ng/mL for sulphadoxine and pyrimethamine, respectively. The ruggedness of the method was confirmed because variations assessed had little effect on separation and quantification of the analytes.
DISCUSSION
In recovery studies, a known amount of analytes was spiked with a determined amount of placebo and the amount of each analyte recovered in relation to the added amount was calculated. The selectivity study revealed the absence of interferences (placebo) since none of the peaks appears at the same retention time of sulphadoxine and pyrimethamine. Hence it was concluded that the developed method is selective in relation to the samples used in this study.8-15
Linearity was demonstrated at concentrations from 10 to 300 µg/mL for sulphadoxine and 10 to 300 ng/mL for pyrimethamine. The correlation coefficients (R2) were 0.9957 for sulphadoxine and 0.9974 for pyrimethamine, indicating a high degree of linearity for both sulphadoxine and pyrimethamine calibration curves. Slope linearity test showed that texp is lower than ttables, which indicate the probability that slope be different of zero is elevated for n-2 degrees of freedom at 95 % confidence interval.8-15
Results of precision of the method showed all the RSD values for percentage of both analytes were < 2.0 % (15). Confidence interval individual results indicate that 95 % of analyses are between 78.07 % and 84.57 % for sulphadoxine, and 93.38 % and 100.74 % for pyrimethamine. Means confidence interval indicates than mean concentration both analytes is with 95 % probability between 78.07 % and 84.57 % for sulphadoxine, and 95.90 % and 98.22 % for pyrimethamine with n-1 (10-1) degrees of freedom. This analysis demonstrated the method precision.8-15
The accuracy of the method was confirmed by measurement of recovery by the standard addition method. Analyzed concentrations were 50, 100, 150 mg/mL for sulphadoxine and 50, 100, 150 ng/mL for pyrimethamine. Cöchran and t-Student tests were used for the data analysis. Results of both Cöchran and t-Student tests demonstrated Gexp and texp values were all < Gtables (0.8709; P= 0.05, K= 3, n= 3) and < ttables (2.306), respectively. Recovery of the components ranged from 92.82 to 97.07 %, and the RSD values were all < 5.0 %; this indicates the method enables highly accurate simultaneous analysis of the two analytes.8-15
The HPLC method described for the simultaneous determination of sulphadoxine and pyrimethamine in 100 mL of whole blood spotted on filter paper has been found to be linear, precise, accurate and selective. Moreover, this method is simple, fast and sensitive and can be used for the monitoring of pharmacokinetic and clinical studies.
AKNOWLEDGMENTS
Authors thank at Seccional de Salud de Antioquia and Universidad de Antioquia for supporting this work.
REFERENCES
1. Edstein M. Quantification of antimalarial drugs. I. Simultaneous measurement of sulphadoxine, N4acetylsulphadoxine and pyrimethamine in human plasma. J Chromatogr. 1984;305:502-7.
2. Bergqvist Y, Eriksson M. Simultaneous determination of pyrimethamine and sulphadoxine in human plasma by high-performance liquid chromatography. Trans R Soc Trop Med Hyg. 1985;79:297-301.
3. Eljaschewitsch J, Padberg J, Schurmann D, Ruf B. High-performance liquid chromatography determination of pyrimethamine, dapsone, monoacetyldapsone, sulphadoxine and N-acetylsulfadoxine after rapid solid-phase extraction. Ther Drug Monit. 1996;18:592-7.
4. Edstein M. Quantification of antimalarial drugs. II. Simultaneous measurement of dapsone, monoacetyldapsone and pyrimethamine in human plasma. J Chromatogr. 1984;307:426-31.
5. Edstein MD, Lika ID, Chongsuphajaisiddhi T, Sabchareon A, Webster HK. Quantitation of Fansimef components (mefloquine + sulfadoxine + pyrimethamine) in human plasma by two high-performance liquid chromatographic methods. Ther Drug Monit. 1991;13:146-51.
6. Astier H, Renard C, Cheminel V, Soares O, Mounier C, Peyron F, et al. Simultaneous determination of pyrimethamine and sulfadoxine in human plasma by high-performance liquid chromatography after automated liquid-solid extraction. J Chromatogr B Biomed Appl. 1997;698(1-2):217-23.
7. Green MD, Mount DL, Nettey H. High-performance liquid chromatography assay for the simultaneous determination of sulphadoxine and pyrimetamine from whole blood dried onto filter paper. J Chromatog B. 2002;767;159-62.
8. Quattrocchi OA, De Andrizzi SA, Laba RF. Introducción a la HPLC Aplicación y Práctica. Buenos Aires: Artes Gráficas Farro, SA; 1992. p. 301-28.
9. Center for Drugs Evaluation and Research (CDER). Reviewer Guidance. Validation Chromatographic Methods. 5600 Fishers Lane, Rockville, Maryland 20857. November, 1994. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM134409.pdf
10. US Food and Drug Administration. Guidance for industry: Q2B validation of analytical procedures: methodology. Rockville, MD: Nov, 1996. Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm073384.pdf
11. Eurachem. The Fitness for Purpose of Analytical Methods: A Laboratory Guide to Method Validation and Related Topics; LGC (Teddington) Ltd.: Middlesex, United Kingdom, 1998. Available from: http://www.eurachem.org/guides/pdf/valid.pdf
12. Swartz ME, Krull IS. Validation of Chromatographic Methods. Pharmaceutical Technology Magazine. 1998;104:104-19.
13. Analytical Procedures and Method Validation: Highlights of FDA´s Draft Guidance. LC-GC [Internet]. 2001 [cited 2011 Aug 30];19(1). Available from: http://www.fda.gov/cder/guidance/2396dft.htm
14. Huber L. Validation of analytical methods: Review and Strategy. Waldbronn: Hewlett-Packard GmbH; 1998.
15. Castro M, Gascón S, Pujol M, Sans JM, Pla LV. Asociación Española de Farmacéuticos de la Industria. Validación de Métodos Analíticos. [Monografía]. Madrid: AEFI; 1989.
Recibido: 3 de mayo de 2012.
Aprobado: 24 de mayo de 2012.
Diana Margarita Márquez Fernández. Facultad de Química Farmacéutica, Universidad de Antioquia. Calle 57 No. 53-108, Bloque 2, Laboratorio 131. Apartado Aéreo 1226. Medellín, Colombia. Correo electrónico: dmarquez@farmacia.udea.edu.co