My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Revista Cubana de Farmacia
On-line version ISSN 1561-2988
Rev Cubana Farm vol.48 no.4 Ciudad de la Habana Oct.-Dec. 2014
PRODUCTO NATURAL
Evaluation of calcium and magnesium citrate from Cuban dolomite
Evaluación de citrato de calcio y magnesio obtenido de dolomitas cubanas
DrC. Jorge E. Rodríguez Chanfrau, MSc. Luis Martínez Álvarez, Lic. Addis Bermello Crespo
Centro de Investigaciones y Desarrollo de Medicamentos. La Habana, Cuba.
ABSTRACT
Introduction: calcium and magnesium salts are used as nutritional supplements obtained from natural sources such as dolomite, which is a double complex of calcium and magnesium carbonate. In search of a calcium raw material with greater bioavailability, a process of obtaining calcium and magnesium citrate salt from dolomite deposits was developed.
Objective: to evaluate calcium and magnesium citrate from dolomite.
Methods: chemical and technological analysis, Powder X-ray Diffractometry attenuated total reflection-Fourier transform infrared spectrometry (ATR-FTIR), differential scanning calorimetry and thermogravimetric analysis were all used.
Results: the chemical analysis confirmed the existence of calcium (over 10 %), and of magnesium (4.5 and 5 %) whereas citric acid content was under 3 %, The levels of toxic metals were below the maximum allowable limits for pharmaceutical products. The density values were below those of the dolomite, with high porosity and flow deficit. The X-ray diffractomery indicated that dolomite was transformed into calcium and magnesium citrate salts whereas, the infrared spectra showed the presence of characteristic COO¯, -OH and -CH2 groups of citrates. The differential scanning calorimetry showed that salt had three endothermic peaks at 101.7 ºC, 167.1 ºC y 194.6 ºC and on the other hand, termogravimetry analysis confirmed that 30.9 % of the total mass is lost at temperatures lower than 295 ºC.
Conclusions: the presence of calcium and magnesium citrate salt is corroborated
Keywords: Calcium and magnesium citrate, dolomite, X-ray diffractometry, infrared spectroscopy, differential scanning calorimetry, thermogravimetric analysis.
RESUMEN
Introducción: las sales de calcio y magnesio son utilizadas como suplementos nutricionales y se obtienen a partir de fuentes naturales, dentro de las cuales se encuentra la dolomita, que es un complejo doble de carbonato de calcio y magnesio. En la búsqueda de una materia prima de calcio con mayor biodisponibilidad, ha sido desarrollado un proceso de obtención de sales de citrato de calcio y magnesio a partir de dolomitas.
Objetivo: evaluar el citrato de calcio y magnesio obtenido a partir de dolomita.
Métodos: se emplearon métodos de análisis químicos y tecnológicos, difracción de rayos X, reflexión total atenuada en el infrarrojo medio con transformada de Fourier, calorimetría diferencial de barrido y análisis termogravimétrico.
Resultados: los resultados del análisis químico demostraron la presencia de calcio (superior al 10 %) y magnesio (entre 4,5 y 5 %), mientras que el contenido de ácido cítrico fue menor al 3 %. Los niveles de metales tóxicos estaban por debajo de los límites máximos permisibles para productos farmacéuticos. Los valores de densidades fueron inferiores a las densidades de la dolomita, con la presencia de un elevado porcentaje de porosidad y deficiente flujo. El análisis por difracción de rayos X demostró que la dolomita fue transformada en sales de citrato de calcio y magnesio, mientras que los espectros infrarrojos mostraron que las principales absorciones se corresponden con las de los grupos COO¯, -OH y -CH2, características todas de citratos. Los estudios por calorimetría diferencial de barrido indicaron que la sal presentaba tres transiciones endotérmicas a 101,7 ºC, 167,1 ºC y 194,6 ºC, y el análisis termogravimétrico corroboró que a temperaturas menores de 295 ºC ocurre una pérdida de masa que representa el 30,9 % de la masa total.
Conclusiones: se corrobora la presencia de sal de citrato de calcio y magnesio.
Palabras clave: citrato de calcio y magnesio, dolomita, difracción de rayos X, espectroscopia infrarroja, calorimetría diferencial de barrido, análisis termogravimétrico.
INTRODUCTION
Osteoporosis, a disease affecting several million older people worldwide, is a condition that must be prevented from childhood. Osteoporosis may be prevented by increasing peak bone mass or decreasing the rate of bone loss with aging. Calcium supplementation of the diet could result in a greater peak bone mass and hence could reduce the risk of fracture in later life. It could also reduce fracture risk during childhood, because these fractures are related to low bone mineral density.1-4
Calcium from carbonate and citrate are the most common forms of calcium supplements. Calcium carbonate, the most cost-effective form, should be taken with a meal to ensure optimal absorption. Calcium citrate can be taken without food and is the supplement of choice for individuals with achlorhydria or who are taking histamine-2 blockers or protein-pump inhibitors.5,6 On the other hand, magnesium is an important mineral in the body. It is present in more than 300 enzymatic systems, where it is crucial for energy production and other metabolic functions. The heart, brain and kidneys cannot function without adequate levels of this nutrient.5,7,8
Calcium is an essential nutrient required in substantial amounts, but many diets are deficient in calcium making supplementation necessary or desirable. The absorption of calcium from dietary supplements has been studied by many methods. Some studies have shown the more soluble calcium citrate to be somewhat better absorbed than the relatively insoluble calcium carbonate.9-11
Several obtaining processes of raw materials with high contents of calcium have been reported. A process to obtain calcium and magnesium citrate from dolomite has been developed. This process consists on making react dolomite with citric acid.12 This study aimed to evaluation of calcium and magnesium citrate from dolomite.
METHODS
MATERIALS
Calcium and magnesium citrate salt was obtained at bench scale from dolomite in accordance with the procedure described in our earlier paper.12 Dolomite (GEOMINERA, Cuba) and citric acid (Proquibasa, Spain) were used.
EVALUATION OF CALCIUM AND MAGNESIUM CITRATE SALTS
Chemical evaluation
Calcium and magnesium determination (complexometric method) and citric acid determination (volumetric method) were used according to Rodríguez, et al.13 Moisture content and total ash according to USP were determined.14
Determination of toxic metals and trace minerals
An atomic absorption spectrophotometer (AAS) Pye Unicam (model PU9100X, England) was employed for the analysis of Aluminium, Iron, Mercury, Cadmium, Lead, Nickel, Sodium, Manganese, Copper, Cobalt, Zinc and Silver.15 The samples were dissolved in 0.1 M hydrochloric acid solution. The lamps employed were of hollow cathode type. The standard curve was prepared employing standard solutions of each metal (SIGMA) under the same instrument conditions as the samples. All sample analysis was triplicates.
Silicon and Fluorine were determined by spectrophotometric method according to the norm of the Central Laboratory of Minerals.16,17 While the arsenic was determined according to the USP.14
Bulk and Tapped Density (Hausner´s Ratio and Carr´s Compressional Index)
The density parameters were determined using an appropriate amount of the sample was poured in a 100 mL tared graduated cylinder. The volume was then read directly from the cylinder and used to calculate the bulk density according to the mass/volume ratio. For tap density the cylinder was tapped 1000 times using a tap density analyzer (Erweka SVM1, Germany). The flow rate was measured by a glass funnel with a round orifice of 120 mm, its outlet is separated 100 mm respect to a horizontal surface, and with a wall angle of 45 degrees. The values were used for the calculation of Hausner's ratio and Carr's compressional index.14,18
Powder particle size analysis.
The particle size distribution was measured using a laser scattering particle size analyzer (model LS 230, Beckman Coulter, USA). For measurement, the sample was diluted to less than 0.02 % w/w to prevent multiples scattering effects. The size distribution of sample powders was determined by dispersing them in absolute ethanol and analyzing them with the same laser scattering spectrophotometer. Determinations were done in triplicate. The powder was characterized as the average particle size (μm).
Powder X-ray Diffractometry
The salts were exposed to CuKα radiation (40 kv x 30 mA) in the step-scan mode with increments of 0.2° 2θ in a Philips (model PW 1218, England) wide-angle X-ray diffractometer. The Bragg-Brentano focusing geometry was used, with a 1º incident aperture slip, a 0.15º detector slit and a scintillation counter as the detector. The measuring time was 3 s/step.
Attenuated Total Reflection-Fourier Transform Infrared Spectrometry (ATR-FTIR)
ATR-FTIR spectra were recorded by using an NICOLET spectrometer (model IR100, USA) with ATR (Thunderdome Swap-Top 0074-150) and spherical glass of germanium. The spectra were recorded over a range of 4000-700 cm−1 with a resolution of 4 cm−1
Differential Scanning Calorimetry
Differential scanning calorimetry (DSC) thermograms of calcium and magnesium citrate salts were obtained by using a differential scanning calorimeter (DSC 20, METTLER TC 10, Switzerland). Samples were accurately weighed into aluminium pans and sealed. In this method, a small hole was made at the top of the pan in order to allow the release of moisture. The measurements were performed at a heating rate of 5 °C/min from 30 °C to 550 °C.
Thermogravimetric Analysis
Thermogravimetric analyses (TGA) were performed by using a thermogravimetric analyzer (TG 50, METTLER TC 10, Switzerland). The measurements were carried out at 30 °C to 750 °C at a heating rate of 10 °C/min.
RESULTS
The table 1 show the results of chemical evaluation and determination of toxic metals and trace minerals. The presence of calcium superior at 10 %, magnesium (between 4.5 and 5 %) and citric acid content smaller to 3 %, were demonstrated.
The analysis by atomic absorption of nickel, sodium, manganese, copper, cobalt, zinc and silver showed that the concentration of these trace minerals was smaller than the limits of detection of the method (0.1 p.p.m.). While the concentrations of the other analyzed trace minerals are below 0,1 %. The levels of toxic metals were below the permissible maximum limits (F< 10 μg/g, Cd< 2 μg/g, Pb< 10 μg/g, Hg< 5 μg/g y As< 3 μg/g)14 for pharmaceutical products, while the moisture content are smaller that 5 %.
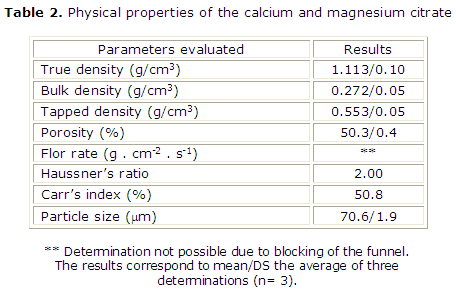
Table 2 show the physical properties of the sample. Concerning the sample density, descending values of true density > tapped density > bulk density were observed. The high values of Hausner's ratio and Carr's index are indicative of an extremely poor flow according to USP14 causing their inability to flow. A high porosity percentage was observed motivated maybe by the packaging type of the solid mass. The particle size was considered appropriate.
The XRD patterns of the sample exhibited peaks at 16.8; 9.4; 8.6; 7.6; 4.82; 4.77; 4.71; 4.64; 3.89; 3.82 y 3.14 Å (Fig. 1). It is observed that the XRD patterns of the sample exhibited peaks similar to the calcium citrate, but lightly displaced. 19 In our opinion this can be due to the presence of magnesium in the compound.
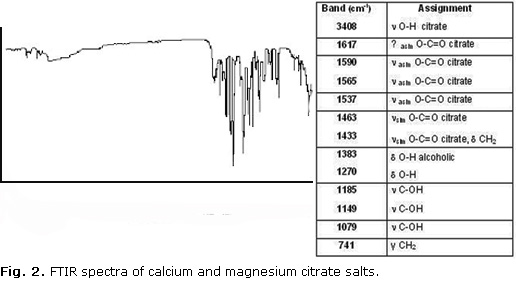
The FTIR spectra of calcium and magnesium citrate salts are illustrated in figure 2. The presence of COO¯, -OH and -CH2 characteristic groups of citrates was observed. The FTIR spectra of the sample exhibited similar bands to those reported in the literature for calcium citrate and magnesium citrate.20,21 In the FTIR spectra of the sample, the bands at 1445 and 727 cm−1 (characteristic to dolomite) and the bands at 1 420, 877 and 714 cm−1 (characteristic to calcite) are not observed.
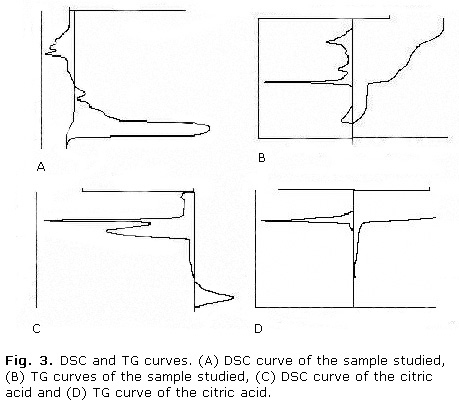
Figure 3 a shows the DSC curves of the sample studied. The DSC curves of the sample are characterized by three endothermic peaks in the temperature range 100-200 °C (101.7 ºC, 167.1 ºC and 194.6 ºC) and a broad exothermic peaks in the temperature 487.2 °C (Fig. 3, A). On the other hand, TG analysis showed that 75.7 % of the total mass was eliminated between 100 and 700 ºC, of them 30.9 % was eliminated to temperatures smaller than 295 ºC (Fig. 3, B).
DSC curve of the citric acid are characterized by two endothermic peaks in the temperature range below to 220 °C (157 ºC and 207 ºC). While, TG analysis showed that 99 % of the total mass is eliminated to same range of temperature (Fig. 3, C and D).
DISCUSION
A powder of white colour, soluble in hydrochloric acid solution and insoluble in water was obtained by technological process developed by Rodríguez, et al.12 Calcium and magnesium are the main components in the sample. On the other hand, the low concentration of trace minerals and toxic elements demonstrate the high purity of the sample.
True density is defined as the density of the material itself; it is the average mass of the particles divided by the solid volume. Bulk density is the mass per unit volume of a loose powder bed. The unit volume includes the spaces between the particles, and the envelope volumes of the particles themselves. Powder bulk density depends primarily on particle size distribution, particle shape, and the tendency of particles to adhere to each other. While, tapped density of a powder is the ratio of the mass of the powder to the volume occupied by the powder after it has been tapped for a defined period of time.22 The density is an essential parameter in the characterization of the powders.
In our study, the results showed that the samples have low bulk and tapped densities but inside the range of most pharmaceutical powders (0.1-0.7 g/mL). 22 These bulk and tapped densities are inferior to densities reported in dolomite samples (bulk densities between 0.972 and 1,097; tapped densities between 1.444 a 1.543).23
Porosity can be derived from powder density, and is an important characteristic of the materials. The high porosity percentage observed was motivated maybe by the packaging type open of the solid mass, indicating the tendency of the material to form agglomerates, affecting the rheology properties of the samples. The flow behavior was faulty. The high values of Hausner's ratio and Carr's index observed in the sample denote their inability to flow.14
Is known the presence of electrostatic charge affect the flow properties of the material. Therefore, the results are logical.24-26 The particle size was considered appropriate.
The XRD patterns and FTIR spectra of the sample are different to the XRD patterns and FTIR spectra of the dolomite.23 This is important because the transformation of the mineral in citrate is demonstrated.
The citrate salts to smaller temperatures of 200 ºC are characterized by the presence of one endothermic peak due to processes of dehydration.21 Besides the endothermic transition of the water, the analyzed sample presents two endothermic transitions due to the citric acid presence. The analysis for DSC and TG of the citric acid sample corroborates these results. A process of dehydration of the sample and decomposition of the present citric acid in the sample were corroborated by means of the thermal study
In conclusion, the result of the study shows that the developed technological process transforms the dolomite in calcium and magnesium citrate salt.
REFERENCES
1. Abad Manteca L, Izquierdo E, Andrés M, Vega G, Mendo M, Pérez Castrillon JL. Prevalencia de osteoporosis en pacientes con síndrome coronario agudo. Rev Osteoporos Metab Miner. 2010;2(1):15-22.
2. Blake GM, Fogelman I. An update on dual-energy X-ray absorptiometry. Semin Nucl Med. 2010;40(1):62-73.
3. Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy X-ray absorptiometry study. J Pediatr. 2001;139:509-15.
4. Ma D, Jones G. The association between bone mineral density, metacarpal morphometry, and upper limb fractures in children: a population-based case-control study. J Clin Endocrinol Metab. 2003;88:1486-91.
5. FAO/WHO. Human, Vitamin and Mineral Requeriments. Report of a joint FAO/WHO expert consultation. Cap. 11 (Calcium); Cap. 14 (Magnesium). Bangkok: FAO/WHO; 2001. p. 151-73, 223-33.
6. Sweet MG, Sweet JM, Jeremiah MP, Galazka SS. Diagnosis and treatment of osteoporosis. Am Fam Physician. 2009;79(3):193-200.
7. Ranade VV, Somberg JC. Bioavailability and pharmacokinetics of magnesium after administration of magnesium salts to humans. Am J Ther. 2001;8(5):345-57.
8. Whang R. Magnesium deficiency: Pathogenesis, prevalence and clinical implication. Am J Med. 1987;87:24-9.
9. Hanzlik R, Fowler S, Fisher D. Relative Bioavailability of Calcium from Calcium Formate, Calcium Citrate, and Calcium Carbonate. JPET. 2005;313(3):1217-22.
10. Heaney RP. Factors influencing the measurement of bioavailability, taking calcium as a model. J Nutr. 2001;131:S1334-48.
11. Sakhaee K, Bhuket T, Adams-Huet B, Sudhaker Rao D. Meta-analysis of calcium bioavailability: a comparison of calcium citrate with calcium carbonate. Am J Ther. 1999;6:313-21.
12. Rodríguez Chanfrau JE, Graveran T, Rodríguez I, Díaz I, Roberto Y, Mateus L, et al. Proceso de obtención de citrato de calcio y magnesio a partir de dolomíta. Patente CO1F 1/00; A61K 33/06, A61K 9/00, C07C 51/41. Certificado 22794. 2002.
13. Rodríguez Chanfrau JE, Lagarto Parra A, Bueno Pavón V, Guerra Sardiña I, Vega Hurtado Y. Effectiveness of washout process to obtain calcium and magnesium citrate at bench scale. Rev Cubana Farm. 2010;45(1):5-12.
14. USP. United States Pharmacopoeia 31. US Pharmacopoeia Convention, Inc. Washington DC: USP; 2008. p. 149, 753-7, 1914-7, 2834-8.
15. Agilent Technologies. Flame Atomic Absorption Spectrometry. Analytical Methods. 10ma ed. New York: Agilent Technologies, Inc.; 2012. p. 15-82.
16. L.M.C. Sílice. Técnica de análisis. Laboratorio Central de Minerales "José Issac del Corral". Cuba: MINBAS; 1988.
17. L.M.C. Flúor. Técnica de análisis. Laboratorio Central de Minerales "José Issac del Corral". Cuba: MINBAS;1988.
18. Iraizoz A, Bilbao O, Barrios MA. Conferencias de Tecnología Farmacéutica. La Habana: Editorial ENPES; 1990. p. 111-241.
19. JCPDS-ICDD. X-ray Diffraction Data. International Centre for Diffraction Data. USA. 1996.
20. SDBS. ASTRIO-DB: Spectral database for organic compounds. National Institute of Advanced Industrial Science and Technology. 2008. [citado 23 nov 2013]. Disponible en: http://riodboi.ibase.aist.go.jp/sdbs/
21. Wagner C, Ferrer E, Baran E. Spectroscopic and thermal behaviour of complex compounds useful for magnesium supplementation. Acta Farm Bonaerense. 1999;18(1):5-12.
22. Amidon GE, Secreast PJ, Mudie D. Particle, powder, and compact characterization. In: Oiu Y, Chen Y, Zhang G. Developing solid oral dosage forms, Pharmaceutical theory and practice. Cap. 8. New York: Elservier Inc.; 2009. p. 163-70.
23. Rodríguez Chanfrau JE, Llanes González A, Roberto Y. Physical, chemical physical and technological characterization of a Cuban dolomite deposit for its possible use as a nutritional supplement source. Rev Aliment. 1999;305:61-5.
24. Drusch S, Serfert Y, Schwarz K. Microencapsulation of fish oil with n-octenylsuccinate-derivatised starch: flow properties and oxidative stability. Eur J Lipid Sci Technol. 2006;108:501-12.
25. Abatzoglou N, Simard J. Prediction of segregation tendency occurrence in dry particulate pharmaceutical mixtures: development of a mathematical tool adapted for granular systems application. Pharm Develop Tech. 2005;1:59-70.
26. Där A. Tecnología Farmacéutica. Madrid: Editorial Arcibia; 1979. p. 22-4.
Recibido: 24 de julio de 2014.
Aprobado: 30 de agosto de 2014.
Jorge E. Rodríguez Chanfrau. Centro de Investigaciones y Desarrollo de Medicamentos. BioCubaFarma. Ave 26 No. 1605 CP 10600. Nuevo Vedado, La Habana, Cuba. Correo electrónico: jorge.rodriguez@infomed.sld.cu