My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Vaccimonitor
Print version ISSN 1025-028X
Vaccimonitor vol.20 no.3 Ciudad de la Habana Sept.-Dec. 2011
ARTÍCULOS ORIGINALES
A single dose of live-attenuated 638 Vibrio cholerae oral vaccine is safe and immunogenic in adult volunteers in Mozambique
Dosis única de la vacuna oral de la cepa viva atenuada 638 de Vibrio cholerae es segura e inmunogénica en voluntarios adultos en Mozambique
Hilda María García1*, Ricardo Thompson2**, Rodrigo Valera1, Rafael Fando3, João Fumane2, Ilesh Jani2, Mayelín Mirabal1, Marlene Isabel Armesto1, Mario Songane2, Sonia Luis2, Ana María Nzualo2, Judite Celeste2, Sofía Viegas2, Eduardo Samo Gudo2, Amélia Melembe2, Dulce Bila2, Cynthia Cemá2, Carolina Mabumo2, Luis García1, Bárbara Cedré1, Gemma Año1, Juan Carlos Martínez1, Aleyda Mandarioti1, Juan Lugones4, Domingo González1, Morelia Baró1, Jonathan Hernández1, Arturo Talavera1, Rosa Lidia Solis1, Gustavo Sierra1, Ramón Barberá1, Francisco Domínguez1, Carlos Gutiérrez3, Concepción Campa1, Ivo Garrido2, Jorge Menéndez1
1Instituto Finlay. Centro de Investigación-Desarrollo-Producción de Vacunas. P.O. Box 16017, La Lisa. La Habana, Cuba.email:hgarcia@finlay.edu.cu
2Instituto Nacional de Saúde, Instituição de Investigação do Ministério da Saúde, Mozambique.
3Centro Nacional de Investigaciones Científicas, P.O. Box 6412, Playa. La Habana, Cuba.
4Empresa Farmacéutica "Reynaldo Gutiérrez", Ave. Independencia, km 5 ½, P.O. Box 10800, Boyeros. La Habana, Cuba.
*MSc in Bacteriology and Micology, PhD in Health Sciences.
**DVM, PhD in Epidemiology, Assistant Professor
ABSTRACT
A placebo-controlled randomized, double-blind, clinical trial was carried out to assess the safety, reactogenicity, and immunogenicity of the lyophilized vaccine candidate against cholera derived from the live attenuated 638 Vibrio cholerae O1 El Tor Ogawa strain. One hundred and twenty presumably healthy female and male adult volunteers aged between 18 and 50 years were included. They were from Maputo, Mozambique a cholera endemic area, where, in addition, human immunodeficiency virus (HIV) seroprevalence is from 20 to 30%. A dose of 2 x 10 9 colony forming units (CFU) was given to 80 subjects and other 40 received only vaccine lyoprotectors as a placebo control. Out-patient follow-up of adverse events was carried out during the following 30 days after vaccination. The immune response was evaluated by the estimation of seroconversion rate and the geometric mean titer (GMT) of vibriocidal antibodies in the sera from volunteers that was collected previously, and at days 14 and 21 after immunization. No serious adverse events were reported. The adverse events found in the vaccine group were similar to those of the placebo groups. They were independent from the detection of antibodies against HIV-1, HIV-2, hepatitis (H) A; HC and hepatitis B surface antigen. The presence of helminthes did not modify the incidence of adverse events. The 638 vaccine strain was isolated in 37 (46.25%) vaccinated volunteer's feces. The peak of the GMT of vibriocidal antibodies in the vaccine group was 9056 versus 39 in the placebo group at 14 days with a total seroconversion of 97.4% at 21 days. The 638 vaccine candidate is safe and immunogenic in a cholera endemic region.
Keywords: Live vaccine, attenuated strain 638, clinical trial, cholera.
RESUMEN
Se realizó un ensayo clínico controlado con placebo, aleatorizado y a doble ciego, para evaluar la seguridad, reactogenicidad e inmunogenicidad del candidato vacunal liofilizado contra el cólera de la cepa viva atenuada 638 de V. cholerae O1 El Tor Ogawa. Se incluyeron 120 voluntarios de ambos sexos, aparentemente sanos, entre 18 y 50 años de edad, en Maputo, Mozambique, un área endémica de cólera, donde la seroprevalencia del virus de inmunodeficiencia humana (VIH) es de 20% a 30%. Ochenta sujetos recibieron como tratamiento una dosis oral de 2 x 10 9 UFC del candidato vacunal 638 y otros 40 una dosis del placebo que contenía solo los lioprotectores. Se realizó seguimiento ambulatorio de los eventos adversos durante 30 días. La respuesta inmune se evaluó por medio de la estimación de la tasa de seroconversión y la media geométrica del título (MGT) de anticuerpos vibriocidas en los sueros de los voluntaries, antes y a los 14 y 21 días posteriores a la inmunización. No se reportaron eventos adversos graves. La incidencia de eventos adversos reportados en el grupo que recibió la vacuna fue similar al del grupo placebo. Los eventos adversos encontrados fueron independientes de la detección de anticuerpos contra el VIH-1, VIH-2, hepatitis (H) A, HC y el antígeno de superficie del virus de la hepatitis B. La presencia de helmintos no modificó la incidencia de eventos adversos. La cepa vacunal fue aislada en 37 (46,25%) voluntarios que recibieron la vacuna. A los 14 días el pico de la MGT de anticuerpos vibriocidas en el grupo de la vacuna fue de 9056 frente a 39 en el grupo placebo, con una seroconversión total de 97,4% a los 21 días. Se concluye que el candidato vacunal 638 es seguro e inmunogénico en una región endémica de cólera.
Palabras clave : Vacuna viva, cepa atenuada 638, ensayo clínico, cólera.
INTRODUCTION
Cholera remains as a global threat to public health in developing countries where infrastructure lacks access to water and to a suitable sanitation, and according to the World Health Organization (WHO), this is one of the indicators of social development. During 2006, WHO participated in the verification of 75 outbreaks of diarrheal disease worldwide confirming 46 outbreaks in 28 different countries (1). The 93% of these events was verified in Africa. Most of the outbreaks outside the African continent have not been verified. A trend to the increase of the number of cases is observed in the East African coast and in Southern Africa. Increased numbers of cases were reported in Malawi (4148), Mozambique (6306), Zambia (5360), and Zimbabwe (789) (1).
There have been changes in trends in the epidemiology of the disease for example, cholera epidemics in sub-Saharan Africa are becoming more frequent, larger and more long-lasting than in other areas, and new variant strains of Vibrio cholerae O1 El Tor that produce the classical cholera toxin are replacing the original El Tor in parts of Africa and Asia and cause a more clinically severe disease (2).
Live, orally administered, attenuated vaccine strains of V. cholerae have many theoretical advantages over killer vaccine. A single oral inoculation could result in intestinal colonization and rapid immune response, being not necessary the use of repetitive dosing. Live V. cholerae organisms can also respond to the intestinal environment and immunological protection against wild-type V. cholerae infection (3).
A number of live oral cholera vaccines have been developed to confer protection against cholera caused by both classical and El Tor biotypes of V. cholerae O1, including CVD 103-HgR, Peru-15 and a new live oral attenuated VA 1.3 strain which has been assessed recently, in human volunteers in Kolkata, India (4). However their effectiveness in endemic areas remains uncertain.
A new lyophilized live oral cholera vaccine candidate has been developed in Cuba, containing the 638 attenuated strain as active pharmaceutical ingredient (5). An advantage of this vaccine candidate is that its in vitro and in vivo growth rate was not affected during its pharmaceutical development. In a phase I-II clinical trial carried out in Cuba, where there is no environmental exposure to V. cholerae, to assess safety, reactogenicity, and immunogenicity of 2 X 109 CFU single dose of the live oral 638 cholera vaccine in healthy adult volunteers, the 638 vaccine candidate was safe, well tolerated, and immunogenic (6).
It is important to know the behavior of the 638 vaccine candidate in cholera-endemic areas, for its clinical development. Mozambique, like other African countries is endemic for cholera disease, where there are also many concomitant parasite infestations and enteric bacterial infections which could affect the V. cholerae colonization into the intestinal loops. It is also important to consider the high prevalence of many other infectious diseases like human immunodeficiency-virus (HIV).
This paper shows the results of a randomized double-blind placebo controlled clinical trial to assess safety, reactogenicity, and immunogenicity of a single oral dose of live attenuated oral 638 cholera vaccine, conducted from August 2006 to November 2006, in Maputo, Mozambique.
MATERIALS AND METHODS
Study design. One-hundred and twenty presumed-healthy male and female adult volunteers from 18 to 50 years old, students from the School Teaching Institute and Industrial and Commercial School of Matola, Mozambique were enrolled in a randomized, double blind, placebo-controlled clinical trial, conducted at the Mozambique's National Institute of Health.
Previous to the enrollment each volunteer received wide information about the study and subsequently they gave written informed consent. Individuals with diarrhea, vomiting, stool cultures positive for V. cholerae, ELISA antitoxin and/or vibriocidal cholera serum antibodies titer higher than 200 and 1280 respectively seven days before vaccination, axillary temperature ³ 37.5 ºC at the moment of vaccine feeding, previous history of immunization with cholera vaccine or infection with cholera, any chronic illness that may affect the study, antibiotic or anti malaria administration, gamma globulin or blood derivates treatment 30 days prior immunization, subject under antiretroviral treatment, pregnant woman or breast-feeding and alcoholism were excluded. Eighty out of them were assigned to the vaccine group, while the remaining forty were assigned to the placebo one group.
This clinical trial protocol was approved by the National Committee of Bioethics for Health, Mozambique and the license for the clinical trial was granted by the Mozambique's Ministry of Health. This clinical trial has been registered in the Cuban Clinical Trial Public Registry that is included in the WHO International Clinical Trial Registry platform with the unique ID number: RPCEC00000051.
Vaccine candidate and placebo formulations. Vaccine candidate and placebo were manufactured at Finlay Institute, Cuba. The vaccine candidate vial contains a lyophilized product, which active pharmaceutical ingredient is the 638 live attenuated cholera strain with a single oral dose of 2 X 10 9 colony forming unit (CFU) mixed with several lyoprotectors: skimmed milk (0.06 g), peptone (0.02 g), and sorbitol (0.02 g) (5). This strain has been modified by engineering techniques to be non-toxinogenic (DCTX-hapA::celA) and it was obtained at the National Center for Scientific Research, Cuba (7, 8). The placebo vial only contained the vaccine lyoprotectors. Each vial was reconstituted with 2 mL of natural water ("El Glacial", Havana, Cuba). The antiacid sachet was manufactured and packaged by "Reynaldo Gutierrez" pharmaceutical enterprise, Havana, Cuba. It contained sodium bicarbonate (2.65 g), ascorbic acid (1.65 g), manitol (0.28 g), anhydrous lactose (0.20 g), sodium saccharine (0.015 g), and polyvinylpyrrolidone (0.20 g), was added to 98 mL of natural water and gently mixed with the vaccine or placebo; previously reconstituted with 2 mL of natural water. The resulting suspension was considered an oral dose.
Randomization. Vaccine and placebo vials were packaged and coded at random with identical appearance. The code remained unbroken until the end of the study.
Vaccination. Subjects were randomized in a double-blind way to receive vaccine or placebo. When the vaccine or placebo was about to be fed to volunteers, it was first reconstituted with 2 mL of natural water, then the antiacid was unpacked and added to 98 mL of natural water until it was dissolved, finally both dissolutions were gently mixed. Volunteers were fasted for 90 min before and after vaccine or placebo feeding.
Specimens. All stools excreted by volunteers were collected every three day, during the following 30 days after vaccination to identify and to quantify the attenuated 638 strains V. cholerae O1 El Tor Ogawa (9) until the occurrence of three consecutive negative cultures of V. cholerae in both groups under study. Stool samples were collected seven days before and after administration of the vaccine or the placebo in order to detect helminthes.
Venous blood collections were performed the day of inoculation and 7 days later in order to evaluate any biochemical changes in volunteers after vaccine and placebo administrations. The behavior of the T CD4+ and T CD8+ lymphocytes sub-population, the variation of total lymphocyte, and the HIV viral load in HIV volunteers, was also described in blood samples collected at days 0, 7, 14, and 21 after vaccination. Sera were also collected on days 14 and 21 after treatment to determine the titers of vibriocidal antibodies directed against Ogawa serotype (10, 11).
Clinical surveillance. The incidence of adverse events was followed-up during 30 days after treatment feeding and recorded by a team of clinical researchers. All volunteers were monitored during the first hour and daily until 30 days after immunization to assess reactogenicity, under an active surveillance to detect the occurrence of solicited adverse event, which were defined as those symptoms and signs such as general malaise, headache, fever, borborygmus, abdominal cramps, nausea, vomiting, and diarrhea, which may occur after the treatment feeding and were solicited to report. Diarrhea was defined as the passage of three or more unformed, loose, or liquid stools in 24 hours (h). Vomiting was defined as one or more episodes of emesis. Fever was defined as an axillary temperature ³ 37.5 ºC. Intensity of the majority of adverse events was classified as mild if it did not interfere with daily activities, moderate if it interfered with but did not impair daily activities, or severe if it impaired daily activities. The intensity of diarrhea, vomiting, and fever were classified as follows: Diarrhea was mild if three passages unformed, loose or liquid stools in 24 h, moderate if between 4-6 passages unformed, loose or liquid stools in 24 h or severe if seven or more passages unformed, loose or liquid stools in 24 hours. Vomiting was classified as mild if one passage of emesis in 24 h, moderate if it was 2-5 passage in 24 h with indication of IV fluid or severe if it was 6 or more in 24 h with indication of IV fluid. Fever was classified as mild if temperature in 24 h was between ³37.5-38.0 °C, moderate if it was between >38.0-38.5 °C or severe if it was between >38.5-39.0 °C. The causality analysis of all adverse events was performed following a casualty algorithm before code were broken (12).
Hematological and biochemical laboratory tests. Routine hematology (red blood cells, hemoglobin, hematocrit, leukocytes, differential blood count, platelets), ABO typing, routine microscopic urine examination (red blood cells, leukocytes, epithelial cells), serum creatinine and liver enzymes (aspartate aminotransferase and alanine aminotransferase), were carried out at the study center.
HIV viral load and CD4/CD8 lymphocyte counts . The HIV viral load was assayed by chain reaction technique for real-time polymerase, blood samples were processed by the Kit COBAS TaqMan HIV-1 Test (Roche Diagnostics, Germany) according to manufacturer's protocols. Samples were processed and analyzed using automated Amplink Version 3.0.1 software. The final concentrations of viral RNA were obtained in copies/mL. Lymphocyte sub-populations counts (CD3, CD4, and CD8) were performed by FACScan (Beckton- Dickinson, San Jose, California, USA) using anti-CD3/CD4 and anti-CD3/CD8 conjugated monoclonal antibody beads.
Health workers of an independent religious organization from Mozambique, conducted a private consultation with all persons included in this clinical trial, to know if each one of them, would agree or not to know about their laboratory testing results mainly those related to HIV serology.
Serology. Vibriocidal antibodies titers in sera were determined by means of a colorimetric microassay method using a commercial freeze-dried complement (Pel Freez/31038.100) (10, 11). The titer was defined as the highest serum dilution that inhibited bacterial growth, as determined by visual color examination. Seroconversion rate was defined as the fourfold increase in vibriocidal antibodies titers after the vaccine or placebo administration with respect to the initial titer.
Antibodies against HIV-1, HIV-2, hepatitis A virus (HAV) and hepatitis C virus (HCV), and the hepatitis B surface antigen (HBsAg) were determined by commercial ELISAs (Abbott).
Microbiologic studies. After vaccination, all stools were plated directly onto thiosufate-citratebile-salt-sucrose (TCBS) Vibrio Selective Agar (Merck, Germany) and also inoculated into alkaline peptone water (Merck, Germany) during an overnight incubation before plating onto TCBS agar.
The determination of CFU of V. cholerae strains per g of stool, were made by dispersion of 1 g of each fecal specimen in 1 mL of 0.9% NaCl, serial dilution, and plating onto TCBS agar. Suspicious colonies from TCBS agar were confirmed as 638 strain by mean of the agglutination with specific antisera, by the resistance to polymyxin B, and by the expression of cel A marker gene (8). Considering the previously described immune-modulation that intestinal parasitisms confer over V. cholerae colonization, stools were examined for intestinal parasites using direct microscopy (13).
Statistical analysis. Calculations were performed to determine the size of population by means of the "bpower" and "bpower.sim" functions from the "hmisc" library, R implementation in the S system (14, 15). Seroconversion percentages and the geometrical mean estimation (GMT) of vibriocidal antibodies titers between vaccines and placebos were compared using the Fisher Exact Test and the Mann Whitney Test respectively (16).
RESULTS
Clinical response: The 638 vaccine candidate was well tolerated in general; neither serious adverse events during the clinical follow-up period nor immediate adverse events during the first hour of active surveillance after treatment administration were reported. No significant changes in the hematological and biochemical parameters done to volunteers´ blood samples after the administration of the vaccine or placebo were detected. Three HIV positive volunteers from the vaccine group were found. No significant variations were detected in the HIV individual results of absolute and relative values of both T CD4/CD8 lymphocytes counting and viral load accomplished to serum samples collected at different moments in the 30 days after the administration of treatments.
Anti-HAV was not found in any volunteer previous vaccination, while the incidence of the anti-HCV antibodies was only 2.53%. On the other hand, the HBsAg seroprevalence was very high (34.18%) in the vaccine group, however any symptom or sign of hepatitis was found, either prior vaccination or during the clinical follow-up.
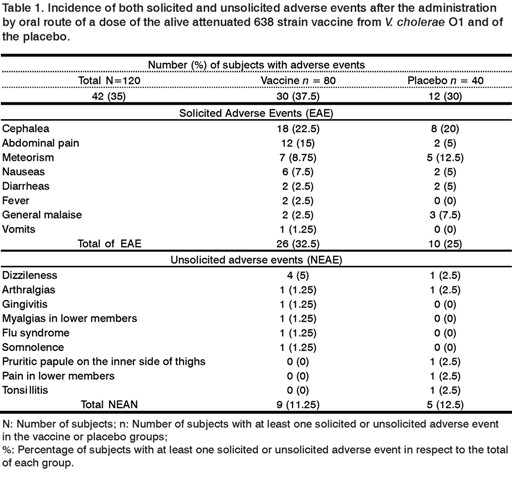
Thirty five percent of subjects involved in the study (42 out of 120), reported various adverse events with similar frequencies in both study groups; 37.5% (30 out of 80) for the group receiving the vaccine and 30% (12 out 40) for placebo group, (Table 1).
Most of the events were mild or moderate and appeared in the first 14 days after vaccination. Only two severe adverse events with less than 24 h occurred. They not needed medicament administration for remission: One was fever with 39.5 ºC in the vaccine group and one abdominal pain in the placebo group.
Regarding solicited adverse events, 32.5% (26 out of 80) was reported in the vaccine group and 25% (10 out of 40) in the placebo group. From these, cephalea was the most frequent adverse event in both groups, 22.5% (18 out of 80) in the vaccine group and 20% (8 out of 40) in placebo group. The abdominal pain appeared in 15% (12 out of 80) in the vaccine group and 5% (2 out of 40) in the placebo group. Meteorism, nauseas, and vomits were less frequent, as well as two diarrheas reported in each group with a short duration, less than 24h.
Most unsolicited adverse events were registered less frequently than solicited adverse events in both groups, 11.25% (9 out of 80) in the vaccine group while 12.5% (5 out of 40) in the placebo one. A case of varicella was additionally registered in the vaccine group after the first 14 days of treatment administration.
Immune response: From the 120 volunteers included in this study, there was one who did not attend to the second serum sample collection (14 days after immunization). Therefore, only 119 were eligible for the immunogenicity analysis.
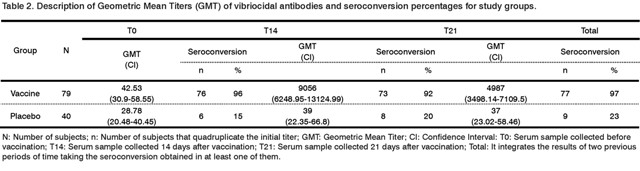
The GMT reached before (T0), at 14 (T14) and 21 days (T21) after the administration of treatments are shown in Table 2. Besides, the results of the seroconversion obtained in the same periods of time are also shown.
The GMT of vibriocidal antibodies reached in the vaccine group was 9056 at 14 days after immunization, while in the placebo group was 39, being the difference of titers reached by each group statistically significant (p<0.001). At 21 days the GMT of vibriocidal antibodies decreased to 4987 in the vaccine group, whereas in the placebo group it remains in 37. In the vaccine group, 84% and 72% of subjects reached vibriocidal antibody titers equal to or greater than 2560 at day 14 and at day 21, respectively, while in the placebo group only one subject reached those titers in the previously mentioned periods of time.
Eight volunteers showed titers of 320 and other six titers of 640 in the vaccine group, before immunization. The rest of volunteers, except one, seroconverted at 14 days after vaccination. Other two volunteers did not seroconverted at days 14 and 21 and there was a third volunteer who did not seroconverted at day 14, but he did it at day 21.
The difference in seroconversion regarding time after vaccination in both groups was also statistically significant (p<0.001), 96.2% and 92.41% at days 14 and 21, respectively, in the vaccine group and 15% and 20% in the placebo group, respectively.
Total seroconversion in the vaccine group was 97.4% and in the placebo group was 22.5%, being this difference between both groups statistically significant (p<0.001). In the placebo group, a total of nine subjects seroconverted and two out of them reached vibriocidal antibodies of 1280 and 10240 at day 14.
No statistically significant differences were found (p=0.7018) between the seroconversion results of volunteers from blood group O (95.92%) and those from other blood groups (96.67%) in the vaccine group.
The three HIV seropositive volunteers seroconverted at 14 and 21 days after the administration of the vaccine, with GMT of 25803 and 4717, respectively.
In the eight subjects from the vaccinated group where helminths were identified is appreciated a GMT of vibriocidal antibodies of 5120 and 2560 at the days 14 and 21 after the treatments administration, reaching a seroconvertion of 100% and 87.5% at the days 14 and 21, respectively.
Bacteriological isolation of the vaccine strain: After the administration of the treatments, none of the volunteers from the placebo group excreted the vaccine strain. The attenuated strain 638 was identified in the feces of 37 out of 80 volunteers from the vaccine group (46%), among which ones are included the 2 volunteers who had diarrhea, and additionally one of the HIV seropositive and six ones in which the helminthes were identified.
The vaccine strain was detected the days 3, 6, and 9 after vaccination with mean concentrations of 1.9x10 6, 9.1x10 6, and 4.6x10 7 CFU, in 17, 33, and 5 volunteers respectively. One volunteer excreted the vaccine strain until the day 15 and other one started and concluded the excretion at the day 9. Seroconversions were registered at days 14 and 21 after administration of treatments, in the 36 volunteers in which the vaccine strain was isolated.
DISCUSSION
The current study, differently from the previous ones (6, 9, 17), involved a larger number of volunteers and it is the first clinical trial with vaccine 638 candidate in a cholera endemic area. Contrary to previous studies performed in Cuba, a country where the disease does not circulate, this trial was carried out at out-patient regime, without prescribing antibiotics, and less restrictive inclusion and exclusion criteria trying to obtain a heterogeneous sample more representative of local population, considered presumably healthy where the prevalence of HIV, hepatitis B, helminthiasis and enterobacteria is high. Under these conditions, the tolerability of the 638 vaccine candidate was demonstrated since neither serious adverse event, nor clinically significant changes in laboratory results after the administration of the vaccine were reported.
A previous clinical trial conducted in Mali to assess safety, reactogenicity, and immunogenicity of a single dose of the CVD 103-HgR in HIV-infected and HIV-non infected adults showed that the serum vibriocidal antibody response was significantly lower in HIV-seropositive subject than in HIV- seronegative subject and much lower in HIV-seropositive with T CD4+ counts below 500 per microliter (18). Other author suggests that the immunization with cholera live vaccines can induce a temporal viral load in HIV-infected, but with only a few clinical alterations or modifications in T CD4+ counts (19). Other data indicate that mucosal immunization with oral cholera vaccine induces a transient increase in HIV viraemia, regardless the clinical infection stage and T CD4+ cell counts. These findings suggest that mucosal stimulation of HIV-infected patients enhances HIV replication (20).
None of HIV subjects whom received 638 vaccine candidate showed clinically important variations in the recounts of CD4/CD8 cells and of the viral load, which reinforces the safety of this vaccine candidate. Nevertheless, obtained data are not comparable with those of other authors (18,19), because the number of HIV positive subjects was lower than the solicited one and the clinical follow-up period was not large enough to detect significant variations in the recounts of T CD4/CD8 cells and of the viral load, describing the individual results in HIV positive volunteers.
As it was solicited, the incidence of adverse events in the vaccine group was indistinguishable from the one found in the placebo group, included both solicited and unsolicited adverse events that might be attributable to the vaccine candidate, which coincides with data reported in other cholera vaccine studies with attenuated strains and applied in endemic areas (21, 22).
The studied population and the site where the clinical assay was conducted have similar characteristics to the ones described by other authors (4, 18, 19, 22). The clinical response to oral cholera vaccines are notably different to the one accumulated in studies performed with populations from non-endemic and more developed areas due to the confluence of different factors such as environmental damage, deficient nutritional status, and concomitant infections by helminths and enterobacteria, among others.
The determination of vibriocidal antibodies in serum is the best assay to assess the antibacterial response to V. cholerae , which is complement-dependent and it is targeted to the lipopolysaccharide (LPS) and associated to protection from infection (23).
Differently to the former clinical trials with fresh culture of the 638 strain and vaccine candidate in Cuba and Ecuador, where antibiotic were given to volunteers 5 day after vaccination to stop vaccine strain shedding and clinical surveillance performed under an inpatient regime, in this study antibiotic were not used, being the clinical surveillance extended till up 30 day after vaccination under an outpatient regime. GMT of vibriocidal antibodies reached 14 days after vaccination under clinical assay conditions in Maputo, were 9056, similar to those reached in patients who had suffered cholera (24), but superior to those reached under pilot clinical assays conducted in Cuba and Ecuador, with GMT of 873 and 896 respectively, as well as those reached in the phase I-II assay with healthy volunteers in Cuba with GMT of 3320. The fact of not administrating antibiotics to volunteers in the study conducted in Maputo, brought about a higher colonization time of attenuated strain 638 in the intestinal tract of volunteers and an increase of GMT after vaccination.
Fourteen out of 120 volunteers from the vaccine group showed pre vaccine vibriocidal antibodies titers of 320 and 640. Thirteen out of those ones did not prevent the adherence and colonization of strain 638 in their small intestine, reaching an elevated immunological response with GMT from 1280 to 40960, at days 14 and 21 after immunization, at a ratio fourfold superior to pre vaccine titers.
Volunteers with positive cholera antitoxin IgG antibodies and with vibriocidal antibodies equal to or greater than 1280 seven days before vaccination were considered as exclusion criteria, because even though the titers of vibriocidal antibodies are not considered surrogate immunity markers, these values are considered disease protective titers in clinical assays and in experimental infection with V. cholerae (24).
It should be noted that the three HIV-seropositive subjects vaccinated seroconverted 14 days after the administration of the vaccine and that they reached vibriocidal antibody titers from 10240 to 40960. This live oral vaccine candidate against cholera might be considered as a control tool in cholera endemic regions, inhabited by subjects coinfected with HIV and V. cholerae . Therefore, it is required to perform clinical trials with an HIV representative population's sample size to confirm our results.
The 638 vaccine strain does not stimulate an antitoxic immune response due to the lacking of the genes coding for subunits A and B of the Cholera Toxin. However, a 10 9 CFU dose of 638 strain to Cuban volunteers, protected them against diarrhea caused by a 10 5 CFU challenge dose of the V. cholerae virulent strain 3008 O1 El Tor Ogawa subsequently administered (17). Therefore, it is possible to hypothesize that the vibriocidal antibody titers (1280-40960) in the vaccinated volunteers co-infected with intestinal parasites in Mozambique, are independent predictors of immunological protection and important markers of protective immunity to cholera infections as reported others (13).
The humoral immune response to the protein antigen CTB (subunit B of cholera toxin) is dependent to the intact population of T CD4+, the immunomodulator effect of the cholera infection or vaccinated patients influences the response development of these cells. Nevertheless, there are not consistent evidences that they may influence the humoral response to LPS, a thyme-independent molecule, main antigen responsible of the antibacterial immune response and in cholera oral vaccine. In addition, the possible influence of helminthes infection driving to a humoral Th2 pattern of immune response has been suggested (13). Any way, additional studies to elucidate these possible interferences need to be addressed.
When comparing GMT values and the seroconversion range reached with our vaccine to those reported for vaccine CVD 103 HgR and to other live oral cholera vaccine candidates in endemic countries, we found out that they are very inferior to those reached by 638 vaccine candidate in presumably healthy adult volunteers in Mozambique (4, 21, 22).
Strain 638 was only recovered in 46% of volunteers from the vaccine group with a mean concentration of 1.9x10 6 -4.6x10 7 CFU/g in fecal excretion. This study shows that strain 638 as active pharmaceutical ingredient in the formulation of this vaccine candidate was able to colonize and adhere to the small intestine of volunteers presumably healthy from a population with poor sanitary conditions and high infestation of common intestinal parasites, without neither increasing its reactogenicity nor occurring secondary severe effects, being stable the reporter gen cel A. The fact that volunteers were followed-up under outpatient regime without interrupting the antibiotic treatment, allowed to know the scope of excretion in one volunteer that excreted the vaccine strain till 15 days and in other one that started and concluded excretion at 9 days, which made possible that 97.4% of volunteers seroconverted at days 14 and 21 after vaccination.
High cholera incidence for 35 years in sub-Saharan Africa and the dynamics of cholera since 2005, together with the apparition of new strains with more serious clinical presentation, the increase of antimicrobial resistance and the weather change suggest that cholera may persist indefinitely.
Vaccination guided and conducted by local health authorities may be effective with the support of local infrastructure after a deep research of historic and current epidemiological situations in well identified geographic areas (25, 26). Additionally, PAHO/WHO by the situation following the recent appearance of the cholera epidemic in Haiti clearly demonstrate the need for an international stockpile of cholera vaccine (27). In this context the 638 vaccine candidate might be used as an auxiliary tool together with other WHO control measures in regions where cholera disease still persist when efficacy be assessed and its sanitary medical registry as pharmaceutical product be achieved.
REFERENCES
1. WHO. Cholera 2006. Weekly Epidemiological Record 2007:82(31):273-84.
2. WHO. Meeting of the Strategic Advisory Group of Experts on immunization, october 2009. Conclusions and recommendations. Weekly Epidemiological Record 2009:84(50):526-8.
3. Ryan ET, Calderwood SB, Qadri F. Live attenuated oral cholera vaccines. Expert Review of Vaccines 2006;5(4):483-94.
4. Mahalanabis D, Ramamurthy T, Nair GB, Ghosh A, Shaikh S, Sen B, et al. Randomized placebo controlled human volunteer trial of a live oral cholera vaccine VA1.3 for safety and immune response. Vaccine 2009; 27:4850-6.
5. Talavera A, Año G, García H, Moreira T, Delgado R, Riverón L, et al. Process development for Cuban cholera vaccine based on the attenuated strain Vibrio cholera e 638. Vaccine 2006;24:3746-9.
6. Valera R, García HM, Díaz Jidy M, Mirabal M, Armesto MI, Fando R. Randomized, double-blind, placebo-controlled trial to evaluate the safety and immunogenicity of live oral cholera vaccine 638 in Cuban adults.Vaccine 2009;27:6564-9.
7. Benítez JA, Silva A, Rodríguez BL, Fando R, Campos J, Robert A, et al. Genetic manipulation of V. cholerae for vaccine development: construction of live attenuated El Tor candidate vaccine strains. Arch Med Res 1996;27:275-83.
8. Robert A, Silva A, Benítez JA, Rodríguez BL, Fando R, Campos J, et al. Taggin a V. cholerae El Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme, Clostridium thermocellone endogluconase A. Vaccine 1996;14:1517-22.
9. Benítez JA, García LG, Silva A, García H, Fando R, Cedré B, et al. Preliminary assessment of the safety and immunogenicity of a new CTX-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infection Immunity 1999;67:539-45.
10. Cedré B, García H, García L, Talavera A. Estandarización y evaluación del ensayo vibriocida. Revista Cubana Medicina Tropical 1999;51:156-9.
11. Cedré B, Viel Y, Rodríguez T, Año G, Pino Y, García H, et al. Validación del ensayo vibriocida colorimétrico para determinar anticuerpos séricos contra cepas candidatas vacunales de V. cholerae. VacciMonitor 2003;12(1):23-30.
12. Peña MA, Valera R, Mirabal M, Rodríguez M, Armesto M, Menéndez J, et al. Propuesta de un algoritmo para evaluar la causalidad de eventos adversos en los Ensayos Clínicos de Vacunas. VacciMonitor 2008;17(3):21-6.
13. Harris JB, Podolsky MJ, Bhuiyan TR, Chowdhury F, Khan AI, LaRocque RC, et al. Immunologic responses to V. cholerae in patients co-infected with intestinal parasites in Bangladesh. PLoS Negl Trop Dis 2009;3(3):e403. Available in:http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2657204/
14.Harrell FE. Hmisc S function library;2003.Available from:http://hesweb1.med.virginia.edu/biostat/s/Hmisc.html
15. R Development Core Team. R:A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. Available from:http://www.R-project.org
16. Agresti A, Coull B. Approximate is better than "exact" for interval estimation of binomial proportions. Am Stat 1998;52:119-26.
17. García L, Díaz Jidy M, García H, Rodríguez BL, Fernández R, Año G, et al. The vaccine candidate V. cholerae 638 is protective against cholera in healthy volunteers. Infect Immun 2005;73:3018-24.
18. Perry RT, Plowe CV, Koumaré B, Bougoudogo F, Kotloff KL, Losonsky GA, et al. A single dose of live oral cholera vaccine CVD 103-HgR is safe and immunogenic in HIV-infected and HIV-noninfected adults in Mali. Bulletin of the World Health Organization 1998;76(1):63-71.
19. Ortigão-de-Sampaio MB, Shattock RJ, Hayes P, Griffin G, Linhares-de-Carvalho MI, Ponce de Leon A, et al. Increase in plasma viral load after oral cholera immunization of HIV-infected subjects. AIDS 1998;12(14):F145-F150.
20. Seidlein L, Wang XY, Macuamule A, Mondlane C, Puri M, Hendriksen I, et al. Is HIV infection associated with an increased risk for cholera? Findings from a case-control study in Mozambique. Tropical Medicine and International Health. 2008;13(5):683-8.
21. Su-Arehawaratana P, Singharaj P, Taylor DN, Hoge C, Trofa A, Kuravanont K, et al. Safety and immunogenicity of different immunization regimens of CVD 103-HgR live oral cholera vaccine in soldiers and civilians in Thailand. J Infect Dis 1992;65:1042-8.
22. Qadry F, Chowdhury MI, Faruque SM, Salam MA, Ahmed T, Begum YA, et al. Randomized, controlled study of the safety and immunogenicity of Peru-15, a live attenuated oral vaccine candidate for cholera, in adult volunteers in Bangladesh. J Infect Dis 2005;192(4):573-9.
23. Holmgren J, Svennerholm AM. Mechanisms of disease and immunity in cholera: a review. J Infect Dis 1977;136(Suppl.1):S105-12.
24. Sahn D, LaRocque RC, Khan AI, Harris JB, Begum YA, Akramuzzaman SM, et al. Incomplete correlation of serum vibriocidal antibody titer with protection from V. cholerae infection in urban Bangladesh. J Infect Dis 2004;189:2318-22.
25. WHO. Cholera 2009. Wkly Epidemiol Rec 2010:85(31):293-308.
26. WHO. Cholera vaccines: WHO position paper. Wkly Epidemiol Rec 2010.85:117-128. Available from: http://www.who.int/wer
27. PAHO. PAHO Expertos hacen un llamado a crear una reserva internacional de la vacuna anticolérica. Disponible en:http://new.paho.org
Received: April 2011
Accepted: May 2011