My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.1 La Habana Jan.-Mar. 2009
RESEARCH
Neuroprotective effect of the systemic administration of MK-801 on the pedunculopontine nucleus of hemiparkinsonian rats
Efecto neuroprotector de la administración sistémica de MK-801 en el núcleo pedunculopontino de ratas hemiparkinsonizadas
Lisette Blanco1, Lourdes Lorigados1, Lisis Martínez1, Nancy Pavón1, María Elena González1, Teresa Serrano1, Vivian Blanco2
1Centro Internacional de Restauración Neurológica , CIREN Ave. 25 No. 15 805 e/ 158 y 160, Playa, Ciudad de La Habana, Cuba
2Centro Comunitario de Salud Mental. Calle 1ra. No. 19 606 e/ 10 y 12, Boyeros, Ciudad de La Habana. Cuba
ABSTRACT
Glutamatergic antagonists were administered in rats, as part of the current pharmacological therapies for neuroprotection of patients with Parkinson disease, due to glutamatergic hyperactivity and the deleterious effects of this condition. The effect of the systemic administration of MK-801, an antagonist of N-methyl-D-aspartate (NMDA) receptors, was evaluated on the extracellular concentrations of glutamate (Glu) and gamma amino butyric acid (GABA), loss of dopaminergic cells and cell death in the pedunculopontine nucleus (PPN) of hemiparkinsonian rats. Five treatments were studied in Wistar rats: lesion in the substantia nigra pars compacta (SNpc) with 6- hydroxydopamine (6-OHDA)(n = 15); 6-OHDA lesion plus the systemic administration of MK-801 (0.5 mg/kg; n = 17); false lesion in the SNpc (n = 10), false lesion in the SNpc plus false systemic treatment (n = 10) and no treatment (n = 22). The extracellular concentrations of Glu and GABA were analyzed by cerebral microdialysis and high performance liquid chromatography coupled to fluorometric detection. The loss of dopaminergic cells and cell death processes in the PPN were assessed by immunohistochemistry for tyrosine hydroxylase and the TUNEL technique, respectively. The extracellular concentrations of Glu and GABA significantly decreased in the PPN after the MK-801 treatment, compared to untreated hemiparkinsonian rats. This treatment caused a decreased loss of dopaminergic cellular bodies in the tegmental ventral area of rats and prevented cell death in the PPN of hemiparkinsonian rats. These results suggest a neuroprotective effect mediated by a decreased glutamatergic tone in hemiparkinsoninan rats systemically treated with an antagonist of NMDA receptors.
Keywords: MK-801, pedunculopontine nucleus, Parkinson disease, 6-OHDA.
RESUMEN
Como parte de las estrategias farmacológicas para la neuroprotección en pacientes con enfermedad de Parkinson se administraron antagonistas glutamatérgicos, ya que se conoce la hiperactividad glutamatérgica en esta afección y su consecuencia deletérea. Se evaluó el efecto de la administración sistémica de MK-801, un antagonista de los receptores N-metil-D-aspartato (NMDA), en las concentraciones extracelulares de glutamato (Glu) y ácido g-aminobutírico (GABA), la pérdida de células dopaminérgicas y el proceso de muerte celular en el núcleo pedunculopontino (NPP) de ratas hemiparkinsonizadas. Se evaluaron cinco grupos de ratas Wistar: ratas con lesión de la substantia nigra pars compacta (SNpc) con 6-hidroxidopamina (6-OHDA) (n = 15), lesionadas con 6-OHDA + administración sistémica de MK-801 (0.5 mg/kg de peso) (n = 17), con falsa lesión de la SNc (n = 10), lesionadas en SNc + falso tratamiento sistémico (n = 10) y ratas sanas (n = 22). Se estudió la concentración extracelular de Glu y GABA mediante microdiálisis cerebral y cromatografía líquida de alta resolución acoplada a detección fluorimétrica, así como la pérdida de células dopaminérgicas y el proceso de muerte celular en el NPP, mediante técnicas inmunohistoquímicas para tirosina hidroxilasa y TUNEL, respectivamente. Las concentraciones extracelulares de Glu y GABA en el NPP disminuyeron significativamente tras el tratamiento sistémico con MK-801, en comparación con las ratas hemiparkinsonizadas que no lo recibieron. La administración de este fármaco provocó menos pérdida de cuerpos celulares dopaminérgicos en el área tegmental ventral de las ratas tratadas, y previno el proceso de muerte celular en el NPP de las ratas hemiparkinsonizadas. Estos resultados sugieren un efecto neuroprotector por la disminución del tono glutamatérgico en ratas hemiparkinsonizadas tratadas sistémicamente con un antagonista de los receptores NMDA.
Palabras clave: MK-801, núcleo pedunculopontino, enfermedad de Parkinson, 6-OHDA.
INTRODUCTION
Parkinson disease (PD) is a disorder affecting dopaminergic and non-dopaminergic structures that progressively degenerate neurons (1). Although the death of the substantia nigra pars compacta (SNpc) dopaminergic cells is the neuropathological signature of PD, together with a subsequent dopaminergic deficiency, the function of other neurotransmitter systems (such as the glutamatergic system), the basal ganglion (BG) and other ganglia are also involved in this disease (2).
PD is currently treated with drugs intended to compensate dopaminergic deficiency, with L-dihydroxy-
phenylalanine (L-DOPA) being the most effective treatment administered (3). Nevertheless, its prolonged use leads to motor and psychiatric complications that have encouraged the search for, and the evaluation of, other neuroprotective pharmacological therapies (4). Those treatments are focused on protecting or preventing the death of the neurons susceptible to the degenerative processes (5, 6).
In this sense, glutamate (Glu) is an excitatory amino acid that activates different types of receptors that are widely distributed throughout BG nuclei (7). In PD, SNpc neuronal loss and the degenerated nigrostriatal network induce hyperactivity in efferent glutamatergic projections of the subtalamic nucleus (STN) towards the globus pallidus medial (GPm), substantia nigra pars reticulata (SNpr), the pedunculopontine nucleus (PPN), the SNpc and the tegmental ventral area (TVA)(8). Thus, Glu becomes an endogenous toxin, provoking the massive entry of calcium into the cells and generating a series of reactions that are detrimental for survival (8).
Experimental evidence show a neuroprotective effect of strategies limiting the action of Glu on its receptors, by administering glutamatergic antagonists, or by attenuating the exposure of SNpc cells to this neurotransmitter through an early lesion of the STN (7-10). Among them, the systemic administration of MK-801 (an N-methyl-D-aspartate receptor antagonist) prevents the toxicity of the direct injection of the 1-methyl-4-phenylpyridinium ion (MPP+) into the SNpc in rats (11). Other studies have documented the protection of SNpc dopaminergic neurons against degeneration, by the concomitant administration of MK-801 and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MTHP) in non-human primates (12).
Most of the studies on neurotransmission and cell death processes in PD experimental models have targeted the defective functioning of the SNpc (8). However, PD-derived changes in other nuclei as the PPN, which is anatomically and functionally related to BG, have been insufficiently investigated (13, 14). PPN is reciprocally connected to the STN through a glutamatergic projection and to the SNpr through a gabaergic projection, both of them are hyperactive in PD (8, 15). Then, both excitatory and inhibitory stimuli converge at PPN from the motor circuitry network, underlying the relevance of studying the extracellular Glu and gamma-amino butyric acid (GABA) concentrations in this nucleus.
The aim of this study was to evaluate the effect of the systemic administration of MK-801 on the extracellular concentrations of Glu and GABA, cell death and loss of nigral dopaminergic cells.
MATERIALS AND METHODS
Experimental subjects
The study used adult male Wistar rats weighing from 200 to 250 g, provided by the Center for the Production of Laboratory Animals (CENPALAB, Havana, Cuba). Three rats were alloted per cage throughout the experiment, with a 12 h/12 h cycle of light and darkness, and water and food were offered ad libitum. The experimental procedures followed the Guideli-nes for the Care, Use and Reproduction of Laboratory Animals.
The SNpc lesion
The animals were anesthetized by the intraperitoneal (i.p.) administration of a chloral hydrate solution (420 mg/kg of body weight) and placed on a stereotactic surgery device for rodents (Stoelting, U.S.A). Three microliters of a neurotoxic solution containing 8 mg of 6-OHDA in 3 µL of 0.9% physiological saline solution (PSS) plus 0.5 mg/mL of ascorbic acid were injected using a flow of 1 mL/min into the right SNpc, at the following stereotactic coordinates (mm) described in the Paxinos and Watson atlas (16): AP = -4.9, L = 1.7, DV = 8.1 (according to Bregma). A control group with false SNpc lesions was obtained by the administration of an identical volume of PSS at the same coordinates.
Animal groups were formed according to the experimental treatment: the SNpc lesion (n=15), the SNpc lesion and the systemic administration of MK- 801 (n=17), the SNpc lesion and the systemic administration of PSS, the false SNpc lesion (n=10) and healthy rats (n=22).
Rotational activity
One month after the injection of 6-OHDA, the rotational activity induced by D-amphetamine (5 mg/kg of body weight, i.p. route) was studied. Only the animals showing at least 7 turns per minute were included in the study. This variable was evaluated for 90 min using a LE 3806 electronic multicounter coupled to LE 902 sensors (PanLab, Barcelona, Spain) that detect the sense of rotation.
Systemic administration of MK-801
The rats received 3 i.p., daily injections of MK-801 (Sigma, St. Louis, USA; 0.5 mg/kg body weight), the third injection was immediately followed by surgery and the injection of the 6-OHDA solution. This schedule was repeated on days 14 and 21 after 6- OHDA administration. MK-801 was dissolved in 0.9% PSS. Control rats received the same treatment, but with PSS instead of MK-801.
In vivo microdialysis
Two weeks after the behavioral studies, a guide cannula was surgically implanted at the coordinates (mm) corresponding to the right PPN (AP = -8.00, L = 2.00, DV = 5.40; according to Bregma). The cerebral microdialysis experiments were performed 24 h after the implantation of this guide. Each rat was connected to a cerebral microdialysis infusion pump (CMA 100, CMA Microdialysis, Stockholm, Sweden) and the cannulae were then continuously perfused, at a flow of 2 µL/min, with a solution of artificial cerebrospinal fluid (aCSF) containing 125 mM NaCl, 2.5 mM KCl, 0.5 mM NaH2PO4, 5 mM Na2HPO4, 1 mM MgCl2 x 6H2O, 1.2 mM CaCl2 and 1.2 mM ascorbic acid, at a pH of 7.4 to 7.6. All the experiments were carried out while the animals were wide awake, as previously described (17).
Biochemical evaluation. Amino acid quantification
Amino acid concentrations in the dialysates were measured by High Performance Liquid Chromatography (HPLC) coupled to a fluorescence detector, via derivation with (OPA). Samples were analyzed in duplicates and procedures were carried out as previously described (17).
Evaluation of dopaminergic cell loss
After concluding the in vivo studies, the rats received a higher dose of chloral hydrate (480 mg/kg body weight, i.p.) and were then perfused via the ascending aorta with 500 mL of 0.9% NaCl and 500 mL of a fixing solution containing 4% paraformaldehyde, 0.1% glutaraldehyde and 15% picric acid in 0.1 M sodium phosphate, pH 7.4. The brains were then extracted, placed in fixing solution for 1 h, washed with 0.1 M sodium phosphate pH 7.4, cryoprotected in 7, 15 and 30% sucrose (24 h at each concentration), and frozen in liquid nitrogen. Coronal sections (20 mm) were obtained from the areas corresponding to the SNpc and placed on microscopy slides previously coated with gelatin-chrome alum. The immunohistochemical processing to visualize cells that are immunoreactive to the tyrosine hydroxylase (TH) enzyme was carried out as described elsewhere (18).
Methodology for the in situ detection of cell death (TUNEL)
The in situ detection of DNA fragmentation was performed using a kit that contains terminal deoxynucleotidyl transferase (TdT) according to the instructions from the manufacturer (Roche Molecular Biochemicals, Mannheim, Germany). Experimental procedures were carried out as previously reported (17).
The occurrence of cell death was determined by contrasting sections with the pentahydrated Hoechst 33258, pentahydrate (bis-benzimide) - FluoroPure grade (Molecular Probes, USA). The sections were examined under a fluorescence microscope (excitation from 500 to 560 nm, detection from 515 to 565 nm; Leitz, Germany).
Data processing
The normal curve followed by the data was verified with the Kolmogorov-Smirnov test in every case. The homogeneity of variance was checked by the Levene test. Comparisons of Glu and GABA concentrations between experimental groups were done by a oneway ANOVA, followed by Tukeys test. The level of statistical significance of 0.05 was chosen for all analyses. The statistical software package Statistica CSS version 6.1 was employed throughout the study.
RESULTS
Extracellular Glu and GABA concentrations at PPN
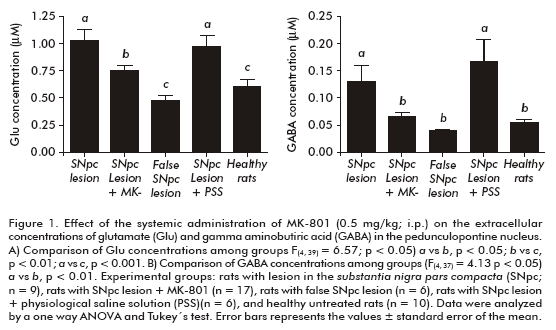
The comparison of the extracellular Glu concentrations revealed statistically significant differences among the experimental groups (F(4, 39) = 6.57, p < 0.05), with the highest levels in the untreated hemiparkinsonian rats. The animals systemically administered with MK-801 showed an intermediate and significantly different behavior (p < 0.05), compared to untreated and SNpc-lesioned rats and to healthy control animals (Figure 1A).
Similarly, extracellular GABA concentrations were significantly different among experimental groups (F(4, 37)=4.13, p<0.05). Once again, the highest values were detected in untreated hemiparkinsonian rats. Animals systemically treated with MK-801 showed significantly lower GABA concentrations that were statistically similar to those of healthy and false SNpc-lesioned rats (Figure 1B).
Presence of dopaminergic cells in the SNpc of hemiparkinsonian rats
The presence of cell bodies positive to the TH enzyme in the TVA of rats systemically administered with MK-801 demonstrates a decreased loss of dopaminergic cells in this zone, compared to untreated rats lesioned in the SNpc (Figure 2A and B). Nevertheless, the pharmacological treatment failed to protect them from loosing dopaminergic cells at the SNpc.
Study of cell DNA fragmentation at PPN
The TUNEL cellular fragmentation study showed TUNEL+ cells in the PPN of untreated hemiparkinsonian rats (Figure 3). On the other hand, the PPN of MK-801-treated hemiparkinsonian rats were weakly immunopositive to TUNEL, suggesting that this treatment prevents the development of cell death processes, but this effect is ipsilateral to the 6-OHDA injection (Figure 3).
DISCUSSION
Considerations on the MK-801 administration schedule used in this study
Our results indicate that the administration schedule followed for the systemic delivery of MK-801 modifies the parkinsonian characteristics generated after the intracerebral injection of 6-OHDA, in agreement with previous reports (19, 20). In general, hemiparkinsonian rats treated with MK-801 showed the poorest loss of dopaminergic cellular bodies in the TVA together with the lowest extracellular concentrations of Glu and GABA and cell death processes at the PPN.
This schedule corresponds to two effects reported for MK-801 in the literature: increased striatal expression of dinorphins (19) and modified discharge frequency of subthalamic glutamatergic neurons (20).
The administration of MK-801 prior to the 6-OHDA injection follows the principle of enhancing the striatal gene expression of dynorphins in neurons of the direct network of the motor circuitry (19). This effect would prevent the decreased striatal-nigral transmission that was established early in the 6-OHDA model (19, 21).
Nevertheless, the behavior during approximately 4 weeks described for the neurotoxic effect of 6-OHDA on nigral cells leads to the establishment of an irreversible motor asymmetry that characterizes this model (21). During that period of time, glutamatergic activity increases gradually, the subthalamic glutamatergic activity peaking at day fifteen and preceding the appearance of PD symptoms (22, 23). The second and third MK-801 administrations pursue the blockage of subthalamic hyperactivity in a critical period of subthalamic activation (24).
Effects of MK-801 administration on the loss of dopaminergic cells
Our results suggest that the MK-801 treatment can protect dopaminergic cell bodies in the TVA from neurotoxic damage. The TVA is part of the mesolimbocortical dopaminergic system and projects the accumbens nucleus (25). There are several reports in the literature showing that the injection of 6-OHDA in coordinates targeting the SNpc similarly compromises the survival of TVA dopaminergic cells (26). This contrasts with the effect of this neurotoxin when administered in the striatum (St) or in the nigral-striatal network where it produces its own retrograde transport, selectively killing SNpc dopaminergic cells and preserving TVA cells (27).
It is well known that at least some of the cascades of neurochemical changes triggered by dopaminergic denervation in BG are reverted by the administration of glutamatergic antagonists (28). Moreover, the expression of NMDA receptors in TVA cells is higher than in SNpc cells, being this difference the possible morphophysiological support for the neuroprotective effect of MK-801 in these cells (29).
The reduced loss of dopaminergic cellular bodies in the TVA of rats treated with MK-801 can significantly influence the maintenance of St under dopaminergic control, at least partially (28). This could attenuate synapse remodeling at St, ipsilateral to the lesion, and consequently decrease locomotor asymmetry in hemiparkinsonian rats (30, 31).
Extracellular Glu and GABA concentrations at the PPN
The decreased extracellular concentrations of Glu and GABA in rats treated with MK-801 after the 6-OHDA injection could result from the treatment by the attenuation of corticostriatal glutamatergic activity and the correction of misbalances of other neurotransmitters as dopamine.
The nigral dopaminergic network and cortical glutamatergic terminals converge at the striatal neuronal projection in a very fine synaptic interaction (32, 33). In PD, a misbalanced estriatal neurotransmission and an increased cortical-striatal glutamatergic transmission coexist (32, 34). Both effects convey, increasing the discharge of action potentials by striatal cells when depolarized after receiving the discharge through the presynaptic cortial-striatal terminal (35).
On the other hand, morphological studies show that dopaminergic denervation leads to structural changes at St, such as the remodeling of dendritic spines, which are able to interfere with cortical-striatal synaptic plasticity mechanisms (36, 37). The systemic administration of antagonists of the NMDA glutamatergic receptors have multiple effects on the functioning of the BG nuclei (38). However, there were no previous references on the effect of the MK-801 administration in the 6-OHDA model, specifically on Glu and GABA neurotransmission at PNN. Hence, our results are a contribution to this field of knowledge.
It was recently shown that the systemic administration of MK-801 reduces the rate of discharge and changes the pattern of electric activity of STN glutamatergic cells in hemiparkinsonian rats (22). It is known that when locally administered in St, MK-801 generates distinct effects depending on the location of the intrastriatal injections and the topological distribution of NMDA receptors within this structure (38). Subsequently, the perfusion of MK- 801 into projection neurons of the St-GP1 network could generate PD-like effects by inhibiting this circuit. In contrast, MK-801 produces marked anti-parkinsonian effects when injected in neurons that originate the St-SNpr network (38).
It has been also reported that the excess of gabaergic inhibition in the PPN in PD can explain the PDassociated hypokinesia, based on the PPN projections through the reticulospinal tract towards the spinal cord interneuronal network (39).
Based on these previous findings, we speculate that the systemic administration of MK-801 used in this study could correct some of the effects of dopaminergic denervation on the corticostriatal excitatory neurotransmission. This will compensate the misbalance among BG motor circuitry networks. Thus, decreased extracellular concentrations of Glu and GABA in the PPN, where both motor circuits converge, may be a functional expression of such a correction.
Cell death at PPN
It is established that approximately 40 to 50% of PPN cholinergic cells are lost in PD (40). In contrast, other authors have pointed out the absence of cell death processes in murine and non-human primate PD experimental models (41). These latter findings have suggested that those neurons are less sensitive than dopaminergic SNpc cells to the exotoxicity processes triggered by the subthalamic glutamatergic activation (38). Nevertheless, there are several, neurochemically different neuronal populations in the PPN. Besides, glutamatergic, gabaergic and peptidergic neurons have been identified at PPN in rats, in addition to the cholinergic ones (14, 42).
In previous studies, we demonstrated the occurrence of one cell death process at PPN, ipsilateral to the 6-OHDA injection (18). The PPN neurons are exposed to an increased subthalamic glutamatergic activity in PD, together with a decreased nigropontine dopaminergic influence (43). This misbalance could be associated to cell death-promoting events in some of these neuronal populations.
The results shown herein suggest that the treatment with MK-801 prevents cell death at PPN. This finding underline the relevance of therapeutic strategies intended to attenuate the glutamatergic hyperactivity on those nuclei innervated by such projections from the STN. Two main mechanisms are expressed in the literature as mediating this effect of MK-801: i) the direct blockage of the NMDA glutamatergic receptor; and ii) the inhibition of the tumor necrosis factor alpha, which enhances Glu toxicity and further inhibits recapture mechanisms of this neurotransmitter (10).
Due to the relevance of the PPN for controlling the voluntary movement of several parts of the body, the effect of the systemic blockage of the glutamatergic transmission on the cell death process is highly relevant. This control is exerted in close association with other brain nuclei (14). Other studies highlight the role of PPN glutamatergic neurons to initiate conscious movements, while the activity of cholinergic neurons is associated to a steady and uniform walking pattern (44, 45).
In a recent study, 36% of the cholinergic and 27% of the non-cholinergic neurons were found to be lost in the PPN of PD patients (46). Data also showed its highly significant negative correlation with the Hoehn and Yahr scores evaluating the axial symptoms of the disease, and its equilibrium and walking disorders. This means that the worse the handicap of the PD patient, the more profound the profile of cell death in the PPN (46).
Thus, cell death could be enhancing PPN malfunction in PD, being part of the morpho-physiological causes of motor disorders found in 6-OHDA-injected hemiparkinsonian rats.
REFERENCES
1. Braak H, Ghebremedhin E, Rüb U, Bratzke H, Del Tredici K. Stages in the development of Parkinsons disease-related pathology. Cell Tissue Res 2004;318:121-34.
2. Muller T, Funchs G, Hahne M, Klein W, Schawarz M. Diagnostic aspects of early Parkinsons disease. J Neurol 2006;253 Suppl 4:29-31.
3. Singh N, Pillay V, Choonora YE. Advances in the treatment of Parkinsons disease. Prog Neurobiol 2007;81:29-44.
4. Van Laar T. Parkinsons disease-related Pharmacotherapy. In: Wolters EC, Van Laar T, Berendse HW (eds.). Parkinsonism and related disorders. Amsterdam: Ed. VU University Express 2007; p. 19-25.
5. López-Lozano JJ, Álvarez-Santullano M. Antiglutamatérgicos, anticolinérgicos y terapias neuroprotectoras en la enfermedad de Parkinson. En: Tolosa ES, Obeso JA, Grandas FJ (eds.). Tratado sobre la enfermedad de Parkinson. 3ra. Edición. Madrid: Ed. Grupo Brystol-Myers Squibb 2004; 85-412.
6. Levi MS, Brimble MA. A review of neuroprotection agents. Curr Med Chem 2004;11(18):2383-97.
7. Alonso-Solís R. Neurotransmisores y neuromoduladores. In: Tresguerres JAF (ed.). Fisiología humana. Madrid: Ed. Interamericana McGraw-Hill 1992; 3-73.
8. Obeso JA, Rodríguez-Oroz MC, Rodríguez M, Lanciego JL, Artieda J, Gonzalo N, Olanow W. Pathophysiology of the basal ganglia in Parkinsons Disease. TINS 2000;23(10):3-18.
9. Blandini F, Greenamyre JT, Fancellu R, Nappi G. Blockade of subthalamic glutamatergic activity corrects changes in neuronal metabolism and motor behavior in rats with nigrostriatal lesions. Neurol Sci 2001;22:49-50.
10. Youdim MBH, Geldenhuys WJ, Van der Schyf J. Why should we use multifunctional neuroprotective and neurorestorative drugs for Parkinsons disease? Parkinsonism Relat Disord 2007;13:S281-S291.
11. Turski L, Bressler K, Retting KJ, Lochsmann P, Watchel H. Protection of substantia nigra from MPP+ neurotoxicity by NMDA antagonist. Nature 1991;349:414-8.
12. Zuddas A, Oberto G, Vaglini F, Fascetti F, Formai F, Corsini G. MK-801 prevents MPTP-induced parkinsonism in primates. J Neurochem 1992;59:733-9.
13. Mena-Segovia J, Bolam JP, Magill PJ. Pedunculopontine nucleus and basal ganglia: distant relatives or part of the same family? TINS 2004;27(10):585-8.
14. Hamani C, Stone S, Laxton A, Lozano M. The pedunculopontine nucleus and movement disorders: Anatomy and the role for deep brain stimulation. Parkinsonism Relat Disord 2007;13:S276-S280.
15. Jenkinson N, Nandi D, Muthusamy K, Gregory R, Stein J, Aziz T. Anatomy, physiology and pathophysiology of the pedunculopontine nucleus. Mov Disord 2009;24(3):319-28.
16. Paxinos G, Watson C. The rat brain in stereotaic coordinates. Academic Press, NY, 1998.
17. Blanco L, Lorigados L, Orozco S, Luisa LL, Pavón N, González ME, et al. Aumento de las concentraciones extracelulares de aminoácidos neurotransmisores y muerte celular en el núcleo pedunculopontino de ratas hemiparkinsonizadas por inyección de intracerebral de 6-hidroxidopamina. Biotecnol Apl 2007;24:33-40.
18. Blanco L, Lorigados L, García R, Martínez L, Pavón N, González ME, et al. Efecto neuroprotector de la administración sistémica de (-) nicotina en ratas hemiparkinsonizadas. Biotecnol Apl 2008;25:126-34.
19. Campbell BM, Kreipke CW, Walker PD. Failure of MK-801 to suppress D1 receptor-mediated induction of locomotor activity and striatal preprotachylinin mRNA expression in the dopamine-depleted rat. Neurosci 2006;137:505-17.
20. Allers KA, Bergstrom DA, Ghazi LJ, Kreiss DS, Walters Jr. MK-801 and amantadine exert different effects on subthalamic neuronal activity in a rodent model of Parkinsons Disease. Exp Neurol 2005;191:104-18.
21. Robinson TE, Mocsary Z, Camp DM, Whishaw IQ. Time course of recovery of extracellular dopamine following partial damage to the nigrostriatal dopamine system. J Neurosci 1994;14(5):2687-96.
22. Bezard E, Boraud T, Bioulac B, Gross C. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci 1999;11:2167-70.
23. Vila M, Périer C, Féger J, Yelnik J, Faucheux B, Ruberg M, et al. Evolution of changes in neuronal activity in the subthalamic nucleus of rats with unilateral lesion of the substantia nigra assessed by metabolic and electrophysiological measurements. Eur J Neurosci 2000;12:337-44.
24. Koutsilieri E, Riederer P. Excitoxicity and new antiglutamatergic strategies in Parkinsons disease and Alzheimers disease. Parkinsonism Relat Disord 2007;13:S329-31.
25. Björklund A, Lindvall O. Dopamine-containing system in the CNS. In: Björklund A, Hökfelt T (eds.). Handbook of chemical neuroanatomy. Vol. 2: Classical transmitter in the CNS. Amsterdam: Ed. Elsevier 1984; 5-122.
26. Blum D, Torch S, Lambeng N, Nissou M, Benabid A, Sadoul R, et al. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinsons disease. Prog Neurobiol 2001;65:135-72.
27. Schober A. Classic toxin-induced animal models of Parkinsons disease: 6-hydroxydopamine and 1-methyl-4- phenyl-1,2,3,6-tetrahydropyridine. Cell Tissue Res 2004;318:215-24.
28. Vila M, Marín C, Ruberg M, Jiménez A, Raisman-Vozari R, Agid Y, et al. Systemic administration of NMDA and AMPA receptor antagonist reverses the neurochemical changes induced by nigrostriatal denervation in basal anglia. J Neurochem 1999;73:344-52.
29. Mathé J, Nomikos G, Hygge K, Svensson T. Differential actions of dizocilpine (MK-801) on the mesolimbic and mesocortical dopamine system: role of neuronal activity. Neuropharmacology 1999;38:121-8.
30. Zigmond MJ, Stricker EM. Parkinson´s disease: studies with an animal model. Life Sci 1984;35:5-18.
31. Metz GA, Whishaw IQ. Drug-Induced Rotation intensity in unilateral dopamine-depleted rats is not correlated with end point or qualitative measures of forelimb or hindlimb motor performance. Neurosci 2002;111(2):325-36.
32. Calabresi P, Centonze D, Bernardi G. Electrophysiology of dopamine in normal and denervated striatal neurons. TINS 2000;23(10):S57-60.
33. Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neurosci 1998;86(2):353-87.
34. Deutch AY. Striatal plasticity in parkinsonism: dystrophic changes in medium spiny neurons and progression in Parkinson´s disease. J Neural Transm Suppl 2006;70:67-70.
35. Groenewen HJ. The basal ganglia and motor control. Neural Plast 2003;10(1-2):107-20.
36. Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat Disord 2007;13:S251-8.
37. Chase Th, Justin D. Striatal dopamine and glutamate- mediated dysregulation in experimental parkinsonism. TINS 2000;23(10):86-90.
38. Nash JE, Brotchie JM. Characterization of striatal NMDA receptors involved in the generation of parkinsonian symptoms: intrastriatal microinjection studies in the 6-OHDA-lesioned rats. Mov Disord 2002;17:455-66.
39. Stein J. Akinesia, motor oscillations and the pedunculopontine nucleus in rats and men. Exp Neurology 2009;215:1-4.
40. Hirsch EC, Graybiel AM, Duyckaerts C, Javoy-Agid F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc Natl Acad Sci USA 1987;84:5976-80.
41. Heise CE, Teo ZCh, Wallace BA, Ashkan K, Benabid AL, Mitrofanis J. Cell survival patterns in the pedunculopontine tegmental nucleus of MPTP monkeys and 6-OHDA-lesioned rats: evidence for differences to idiopathic Parkinson ´s disease patients? Anat Embryol 2005;210:287-302.
42. García-Rill E. The pedunculopontine nucleus. Prog Neurobiol 1991;36:363-89.
43. Schulz JB. Mechanisms of neurodegeneration in idiopathic Parkinsons disease. Parkinsonism Relat Disord 2007;13:S306-8.
44. García-Rill E. The basal ganglia and the locomotor regions. Brain Res 1996;11:4763.
45. Bhidayasiri R, Gasser H, Cohen SN, Tourtellote WW. Midbrain ataxia: possible role of the pedunculopontine nucleus in human locomotion. Cerebrovasc Dis 2002;234:1-6.
46. Rinne JO, Yong Ma S, Sik M, Collan Y, Röytta M. Loss of cholinergic neurons in the pedunculopontine nucleus in Parkinsons disease is related to disability of the patients. Parkinsonism Relat Disord 2008;14(7):553-7.
Received in October, 2008.
Accepted for publication in March, 2009.
Lisette Blanco. Centro Internacional de Restauración Neurológica, CIREN. Ave. 25 No. 15 805 e/ 158 y 160, Playa, Ciudad de La Habana. Cuba. E-mail: lisette.blanco@infomed.sld.cu