My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.2 La Habana Apr.-June 2009
RESEARCH
Influence of the number of animals on the production of monoclonal antibody CB.Hep-1 by the Ascites method
Influencia del número de animales en la producción del anticuerpo monoclonal CB.Hep-1 mediante el método de producción ascitis
Otto Mendoza1, Rodolfo Valdés1, Marcos González1, Rosario Alemán1, Tatiana Álvarez1, Sigifredo Padilla1, Andrés Tamayo1, Biunayki Reyes1, Déborah Geada2, Eutimio G Fernández3, Dasha Fuentes4, Odys Hernández4, Leticia Zuasnabar4
1Monoclonal Antibody Department, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, Cuabanacán, Playa, PO Box 6162, CP 10600, Havana, Cuba
2Tobacco Research Institute, Tumbadero Road km 8½, San Antonio de los Baños 3500, Havana, Cuba
3Center for Engineering and Chemical Research, F Street, # 11518 / Ave. 5th and Calzada, Havana, Cuba
4National Center for Laboratory Animal Breeding, Cacahual Road km 21/2, Bejucal, Havana 3, Havana 10400, Cuba
ABSTRACT
The ascites production method of inoculating hybridoma B cells in histocompatible mice can successfully produce monoclonal antibodies (MAb). The aim of this paper is to study the influence of the number of animals on MAb CB.Hep-1 production by the ascites production method. CB.Hep-1 mouse hybridoma cells were inoculated in different groups of animals (I, 750 mice; II, 1000 mice, III, 3500 mice; IV, 4000 mice and V, 6000 mice) previously irritated with mineral oil. The ascitic fluid was harvested through abdominal paracentesis and the results showed a marked influence of the number of animals on the amount of MAb CB.Hep-1 produced by the mice (I, 15.70 mg; II, 19.74 mg; III, 9.91 mg; IV, 8.46 mg; V, 5.30 mg). In conclusion, the concept of Reduction (3R concepts) was rigorously studied in this paper, determining the lowest number of inoculated animals needed for the highest amount of the MAb CB.Hep-1. Results indicate that the number of mice with tumors and the MAb CB.Hep-1 production decrease with the increase in the number of animals inoculated. Therefore between 50 000 and 250 000 animals could be spared each year if the number of animals was of ≤ 3500 per inoculation under the conditions studied here.
Keywords: Ascites, Monoclonal antibodies, 3R concepts, Mice.
RESUMEN
El método de producción de ascitis mediante la inoculación de hibridomas histocompatibles con los ratones puede producir anticuerpos monoclonales (AcM). El objetivo de este trabajo fue estudiar la influencia del número de animales sobre la producción del AcM CB.Hep-1 mediante la producción de ascitis. Las células del hibridoma de ratón CB.Hep-1 se inocularon en diferentes grupos de animales (I, 750; II, 1000; III, 3500; IV, 4000 and V, 6000) previamente irritados con aceite mineral. El fluido ascítico se cosechó mediante paracentesis abdominal y los resultados demostraron una marcada influencia del número de animales sobre la cantidad de AcM CB.Hep-1 por ratón (I, 15.70 mg; II, 19.74 mg; III, 9.91 mg; IV, 8.46 mg; V, 5.30 mg). En conclusión, el concepto de Reducción (conceptos 3R) fue rigurosamente estudiado en este trabajo, determinándose el mínimo número de animales que permite obtener la máxima cantidad del AcM CB.Hep-1. Los resultados indican que el número de animales con tumor y la producción del AcM CB.Hep-1 disminuyen con el incremento del número de animales inoculados y por lo tanto entre 50 000 y 250 000 animales por año podrían ser salvados si el número de animales inoculados fueran ≤ 3500 por cada inoculación bajos las condiciones estudiadas.
Palabras clave: Ascitis, Anticuerpos Monoclonales, Conceptos 3R, Ratones.
INTRODUCTION
Since the development of monoclonal antibody (MAb) technology, there have been many biomedical and biotechnological applications involving these molecules (1-2). Within the MAb production methods, ascites production has been successfully applied. This procedure is based on histocompatible hybridoma cells inoculation into the peritoneal cavity of mice and the tumor formed further produces MAb rich ascites (3-4).
In vivo MAb production can yield high concentrations of MAbs in a relatively short period of time. But this technique also has several disadvantages. For instance, it requires the facilities for animals, trained personnel and the scaling-up of production is difficult. Additionally, ascitic fluid is a complex mixture of components, therefore, it has the same difficulties associated to the use of serum for in vitro cultures and it may be contaminated with mouse plasma proteins, cytokines, growth factors, immunoglobulins, bacteria and viruses.
Nowadays, this procedure is not the method of choice for MAbs production. However, in vitro methods fail to grow hybridomas in some cases and serum-supplemented in vitro cultures have identical in vivo method downstream purification problems. Additionally, the production of antibodies in transgenic plants and animals show a low level of expression and problems associated to the homogeneity of molecules, respectively. Thus, the use of the ascites method is still needed in some cases.
Also, laboratory animals protection societies, have issued guidelines to regulate the use of animals in biomedical applications (5). The European community banned the massive use of animals for such purposes (6-7). In the United States, large-scale ascites production was also finally restricted using as the main criteria, the protection of animals and the potential loss of the European market (7). The 3R concepts (Replacement, Refinement and Reduction) were first used almost 50 years ago providing a framework to improve the ethical acceptability of experimental techniques in animals. Given that animals used in research may experience pain, suffering or long lasting harm, the first step must be to consider whether less sentient or non-sentient alternatives can be used instead (Replacement). Where this is not possible, care needs to be taken to minimize pain (Refinement). Refinement is often achieved, by providing animals with an environment in which they can feel safe and free from infectious diseases. Finally, the number of animals used in a given project needs to be minimized (Reduction), while ensuring that the purpose of the study is achieved (5, 8-10).
The literature is full of articles on MAb production by the ascites method; however, there is a limited amount of articles on the influence of the number of animals on the production of monoclonal antibodies in ascites. Therefore, this investigation was made to provide evidence on whether the MAb CB.Hep-1 production is affected by the number of inoculated mice, measuring their capacity to develop ascitic tumors, the volume of ascites and MAb concentration, for maximizing MAb yield and consequently reducing the number of animals; whereas new alternatives such as in vitro production or transgenic plants are able to support large-scale MAb CB.Hep-1 production as a valid replacement alternative technique (11-14).
MATERIALS AND METHODS
Hybridoma cell line
The myeloma SP2/0-Ag14-derived hybridoma 48/1/ 5/4 (CB.Hep-1) was obtained using BALB/c mice subcutaneously immunized with the Hepatitis B surface antigen (HBsAg) isolated from a person chronically infected with the Hepatitis B virus (15).
Cell culture medium and additives
The cell culture medium used was RPMI1640 (Gibco- BRL, USA) supplemented with 2 mM L-glutamine, 1 mM sodium piruvate, 17 mM sodium bicarbonate and 25 mg/L gentamicin (Gibco, USA) and 8% FCS (Hyclone, USA).
Hybridoma culture
Cells were cultured in 1 L spinner-flasks, starting from a cell concentration of 3 x 105 cells/mL. Cells were always maintained at 37 °C in a 5% CO2 atmosphere and the cell culture medium was replaced every 48 h until reaching the highest cell density. Cell counts and cell viability were measured using the vital trypanblue reagent (16).
Hybridoma specific growth rate and doubling time determination
The specific growth rate (EGR) and cell doubling time (DT) (17) were calculated as follows:
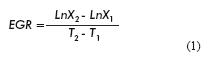
Where:
X1 and X2 are the number of cells alive at time 1 and 2 respectively.
T1 and T2 are the sampling points 1 and 2 respectively.
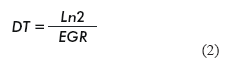
Mouse inoculation and ascites harvest
BALB/c male and female mice of 24 ± 1 and 22 ± 1 g of weight respectively were used for ascitic fluid production. The groups of animals were (I, 750 mice; II, 1000 mice, III, 3500 mice; IV, 4000 mice and V, 6000 mice). They were maintained in TV-3 cages (425 mm x 266 mm x 155 mm) at 22 °C, and 65-80% relative humidity and low levels of ammonium. Animals were primed with 0.5 mL of mineral oil into the abdominal cavity 10 days before cell inoculation. One million cells suspended in one milliliter of RPMI1640 (Gibco- BRL, USA), not supplemented with FCS, were inoculated by intraperitonial injection in each primed BALB/c mouse and the number of animals inoculated by each person never exceeded 300. The study was completely performed under controlled conditions to avoid the influence of temperature and humidity on the animals. After the inoculation of the cells, they were clinically monitored to discard dead animals. The ascites was harvested in 50 mL corning tubes under aseptic conditions inside a sterile hood by abdominal paracentesis (using the right side of the inguinal-abdominal region). Before the ascites extraction, tapping, the abdominal zone of each animal was cleaned with 70% ethanol. After the harvest, it was centrifuged at 3000 rpm for 15 min (Hitachi centrifuge SCR7B, Japan) to separate cells from the liquid phase. A representative group of animals from each population (I-V) were studied by mouse antibody production (MAP), parasitology and microbiology tests to discard the influence of contamination by microorganisms on the health and ascites production of the mice.
Estimation of antibody by enzyme-linked immunosorbent assay (ELISA)
A polystyrene microplate (Costa, USA) was coated with 10 μg per well of the rHBsAg in 100 mM NaHCO3 for 20 min at 50 °C. Then, samples were added to the plate in 0.05% Tween 20/150 mM PBS (phosphate buffered saline solution), and incubated for 1 h at 37 °C. Several washes with 0.05% Tween 20/150 mM PBS were done and subsequently the plate was incubated for 1 h at 37 °C with a horseradish peroxidase conjugate (Sigma Chemical, St. Louis, USA). The reaction was then developed using 100 μL/well of 0.05% O-phenylenediamine and 0.015% H2O2 in citrate buffer; pH 5.0, and stopped with 50 μL/well of 1.25M H2SO4. Absorbance was measured by a Multiskan ELISA Reader (Labsystems, Finland) using a 492-nm filter (18). The range of the calibration curve was 3.13-50 ng/mL. The standard MAb used was the IgG2B070305 supplied by the Center for Genetic Engineering and Biotechnology, Havana, Cuba.
Statistical analysis
The STATGRAPHICS Plus version 5.0, 2000 from Statistical Graphics Corp., USA and Microsoft Excel programs were used for data processing. In all cases, the confidence level employed was 0.05.
RESULTS AND DISCUSSION
The generation of MAbs originally started four decades ago, time in which Khöler and Milstein published a paper describing a new method for producing MAbs (1). Many fields of research such as, biomedicine and biotechnology readily adopted this revolutionary technique, but at the same time, the production of MAbs in mice created problems regarding cost, biological safety, and the care and use of animals (5).
Nowadays, MAb production using animals tends to disappear (19). In some countries, an exceptional justification for MAb production by the ascites method must be given on a case by case basis and failure to produce the specific product after in vitro attempts is an essential requirement for the production of these molecules by the ascites method (5). In vitro methods have been widely developed in the last 20 years and a wide range of in vitro production systems have been developed for different purposes; however there are some hybridomas that do not show a high secretion of MAbs in cell culture.
At the same time, MAb production in transgenic plants seem to be the most promising technology to solve the problems associated with the large demand of these molecules and the limitations of the ascites method. Nevertheless, hybridoma CB.Hep-1 showed a low MAb CB.Hep-1 secretion and stability in cell culture systems using the isolation cell culture medium and also a very poor expression in transgenic tobacco plants (11-13).
Therefore, the replacement of the ascites method for the production of MAb CB.Hep-1 was not yet possible. The main advantage of the ascites method here is the high antibody yield, which generally lies in the range 1-4 mg/mL. Nevertheless, several studies had to be done to demonstrate the optimal number of animals needed.
About fifty years ago, Charles Hume provided a framework for improving the conduct and ethical acceptability of experimental techniques on animals (20). Hence, the main objective of this study was to provide evidence on the influence of the number of animals on the production of MAb CB.Hep-1 so as to reduce this number.
There is an inverse relationship between the amount of hybridoma cells and animal survival (21). Therefore, the lowest number of cells that can produce a significant volume of ascitic fluid and the highest animal survival had been previously studied. Results showed that the inoculation of 1 x 106 cells per animal did not produce differences in ascites volume, MAb concentration and animal survival compared to 3 x 106 and 10 x 106 cells per animal respectively (data not shown). Ten million cells per animal, promoted solid tumor formation in the peritoneal cavity, while cell concentrations lower than 1 x 106 required a three times longer period of time to produce comparable amounts of ascites rich in MAb CB.Hep-1 thereby prolonging the time in which mice were in distress.
The influence of the age of the hybridoma cell on MAb productivity has also been investigated in cell culture (22). However, few reports have been found on the influence of cell age on ascites production kinetics and MAb concentration. To avoid the effect of this variable on MAb CB.Hep-1 production, cell inoculations were made from the same cell stock and were inoculated with a similar number of passages, EGR, DT, cell viability and MAb secretion (Table 1).
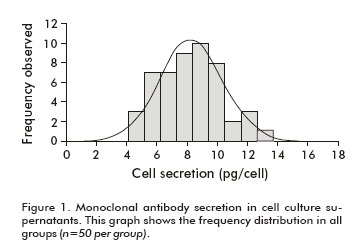
Average cell viability ranged from 95.9 ± 1.8% to 97.9 ± 1.5% for all groups. Thus, all animals were inoculated with about 0.96-0.98 x 106 living cells, which mean that significant differences between groups were not observed (Table 1 and Table 2). MAb secretion level of the cell population showed a quasi similar frequency distribution with an average secretion in a range of 7.5 ± 2.4 pg/cell to 8.9 ± 1.6 pg/cell (Figure 1).
The development of ascitic tumors in mice can be affected mainly by the health condition, the histocompatibility between hybridoma cells and animals, cellular tumorigenicity and the number of cells (3). But, it is important to emphasize that other factors such as humidity, noise, temperature, food, water, environmental ammonium concentration, weight, age, sex and the number of animals could also affect ascitic fluid production.
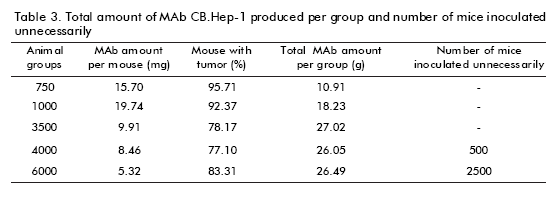
The ability to induce ascitic tumors is an important criterion in scaling-up MAb production, which is not linear, because it determines the total number of animals to be inoculated. Once the CB.Hep-1 cells were inoculated into the mice, the ascitic tumor was observed in most of the mice during seven days. The number of animals with tumors decreased on increasing the number of animals inoculated (Table 1). Groups of 750 and 1000 animals showed an average of over 92% of the animals with tumors. While, the number of animals with tumors ranged from 77% to 83% in the other groups of animals (3500, 4000 and 6000), showing significant differences (p = 0.00006) (Table 2).
The number of abdominal taps per mouse also affects MAb production. This is one of the reasons for criticizing the ascites method on the basis of humane concern. The prolongation of tapping time increases the clinically pathological abnormalities developed in the mice as a result of disseminated or solid tumor growth within the peritoneal cavity and the associated accumulation of ascites (23). Consequently, several countries have established guidelines restricting or prohibiting the massive use of rodents in ascites production (24). In general, these guidelines approve only two or three ascites harvests. However several studies report that up to seven taps may be taken. These aggressive approaches may results in higher MAb yield. The reduction in the number of taps may result in the use of a limited duration of discomfort minimizing pain and distress from the effects of hybridoma growth, ascites development and the tapping procedure itself. Here, we decided to extend the number of taps up to seven, because mice produced well, they did not show signs of weight loss and maintained relative good clinical conditions. Our reasoning was: more taps increase the duration of discomfort but reduce the total number of animals to be used if MAb concentration and volume remain high. It is not recommendable to reduce the number of taps if the total amount of animals suffering will consequently increase (5). A refined study on the estimation of MAb CB. Hep-1 concentration in each tap and the number of animals that could be spared will be shown afterwards.
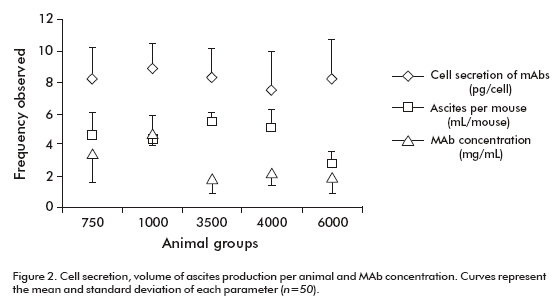
The total volume of ascites from each group was divided by the number of animals with ascites to estimate the volume of ascites produced by each mouse inoculated. The value ranged from 5.5 ± 0.6 to 2.8 ± 0.8 mL per animal, reaching the minimum value in the group of 6000 animals (Table 1, Figure 2). This parameter showed statistical differences between the group of 6000 animals (p = 0.0012) and all other groups according to the Kruskal-Wallis test and the Box and whisker plot (Table 2).
These differences could only be understood on considering the difficulties in handling a large number of animals and/or in the time required to inoculate large amounts of animals provoking cell damage and death. Further experiments should be carried out to determined the true influence of inoculation time on cell viability and ascites production, because although the number of animals per operator was very similar (< 300 mice) the skill of each individual is different. Ascites volume could also be influenced by the deficient priming of animals with mineral oil.
A single tumor-bearing mouse is able to produce 10-20 mg of MAbs (4). Here, a specific ELISA was used to estimate MAb CB.Hep-1 concentration. Data showed that MAb CB.Hep-1 production by mice inoculated with the hybridoma CB.Hep-1 ranged from 5.32 to 15.70 mg/mouse with a clear decrease on increasing the number of inoculated animals (Table 3).
Figure 3 also illustrates the marked influence of the number of animals on MAb concentration. In the groups of 750 and 1000 mice, MAb concentration was higher than that observed in the groups of 3500, 4000 and 6000 mice respectively. According to the Kruskal- Wallis p-value (p = 0.0251) and the Box and whisker plot, there are statistically significant differences between groups, with a 95% confidence level (Table 2). Consequently, the total amount of MAb CB.Hep-1 produced by each group of animals achieved its maximum value, 27.02 g, with 3500 animals (Table 3).
Finally, the data presented here can help in the discussion of the rationale for the use of animals in MAb CB.Hep-1 production, evaluating the number of mice that could be spared if a number of inoculations were demanded for producing MAb CB.Hep-1 to manufacture the Hepatitis B vaccine. Thus, the last objective was to decrease (Reduction) the number of mice inoculated to yield a given amount of MAb CB.Hep-1 through ascites. The cumulative amount of animals inoculated unnecessarily is illustrated in figure 3. A total amount of between 50 000 and 250 000 animals could be spared each year if the number of mice per group were from 4000 to 6000. This type of study can be applied to all Mab production systems in animals to significantly reduce costs and the number of animals, while taking into consideration that other variables such as hybridoma characteristics, animal/ operators ratio, and quality of animals should also be controlled.
CONCLUSIONS
The number of mice with tumors and MAb CB.Hep-1 production decrease with an increase in the number of inoculated animals, thus between 50 000 and 250 000 animals could be spared every year, if the number of animals inoculated with hybridoma CB.Hep-1 were of ≤ 3500 per inoculation under the conditions shown here.
ACKNOWLEDGEMENTS
Authors would like to thank the Gnotobiotic Animal Division at the National Center for Laboratory Animal Breeding, the Process Control Department at the Center for Genetic Engineering and Biotechnology for their assistance in controls, and Leiany M. Cáceres for her assistance in reviewing the manuscript.
REFERENCES
1. Köhler G, Milstein C. Continuous culture of fused cells secreting antibodies of predefined specificity. Nature 1995;256:485-97.
2. Kim J, Roh S, Koo K, Cho Y, Kim H, Yu C, et al. Preclinical application of radioimmunoguided surgery using anti-carcinoembryonic antigen biparatopic antibody in the colon cancer. Eur Surg Res 2005;37(2):36-44.
3. Brodeur BR, Tsang P, Larose Y. Parameters affecting ascites tumor formation in mice and monoclonal antibody production. J Immunol Methods 1984;71:265-72.
4. Brodeur BR, Tsang PS. High yield monoclonal antibody production in ascites. J Immunol Methods 1986;86:239-41.
5. Richmond J. The 3Rs-past, present and future. Scand J Lab Anim Sci 2000;2:84-92.
6. Hendriksen CFM, de Leeuw W. Production of monoclonal antibodies by the ascites method in laboratory animals. 74th Forum in Immunology. Res Immunol 1998;149:535-42.
7. Hendriksen CFM. A call for a European prohibition of monoclonal antibody (MAb) production by the ascites procedure. ATLA 1999;26:523-40.
8. McGuill MW, Rowan AN. Refinement of monoclonal antibody production and animal well-being. ILAR News 1989;31:7-11.
9. Flecknell PA. Refinement of animal use: assessment and alleviation of pain and distress. Lab Anim 1994;28:222-31.
10. Rowan AN. The third R: Refinement. ATLA 1995;23:332-46.
11. Valdés R, Ibarra N, González M, Alvarez T, García S, Llambias R, et al. CB.Hep-1 hybridoma growth and antibody production using protein free medium in hollow fiber bioreactor. Cytotechnol 2001;35:145-54.
12. Valdés R, González M, González Y, Gómez H, García J, Alvarez T, et al. Production of an anti-HBsAg mouse IgG-2bk monoclonal antibody in hollow fiber bioreactors using different cell culture media and operation modes. Biotecnol Apl 2005;22:112-6.
13. Ramírez N, Rodríguez M, Ayala M, Cremata J, Pérez M, Martínez A, et al. Expression and characterization of an anti-(hepatitis B surface antigen) glycosylated mouse antibody in transgenic tobacco plants and its use in the immunopurification of its target antigen. Biotechnol Appl Biochem 2003;38:223-30.
14. Valdés R, Gómez L, Padilla S, Brito J, Reyes B, Alvarez T, et al. Large-scale purification of an antibody directed against hepatitis B surface antigen from transgenic tobacco plants. Biochem Biophys Res Commun 2003;308:94-100.
15. Fontirrochi G, Dueñas M, Fernández de Cossio ME, Fuentes P, Pérez M, Mainét D, et al. A mouse hybridoma cell secreting IgG and IgM antibodies with specificity for the hepatitis B surface antigen. Biotecnol Apl 1993;10:24-30.
16. Patterson MK. Measurement of growth and viability of cells in culture in Jacoby WB, Pastan IH, editors. Meth Enzymol. Academic Press, London:1973. p. 58.
17. Merten O-W. Batch production and growth kinetics of hybridomas. Cytotechnol 1988;1:113-21.
18. Leyva A, Franco A, González T, Sánchez J, López I, Geada D, et al. A rapid and sensitive ELISA to quantify an HBsAg specific monoclonal antibody and a plant-derived antibody during their downstream purification process. Biologicals 2007;35:19-25.
19. Jackson LR, Trudel LJ, Fox JG, Lipman NS. Monoclonal antibody production in murine ascites I. Clinical and pathologic features. Lab Anim Sci 1999;49:70-80.
20. Hume CW. The Legal Protection of Laboratory Animals in: The UFAW Handbook on the Care and Management of Laboratory Animals. 2nd ed. Worden and Lane-Petter: UFAW, London; 1957. p. 65-78.
21. De Deken RJ, Brandt F. Influence of the priming and inoculation dose on production of monoclonal antibodies in two age groups of BALB/c mice. Hybridoma 1994;12:53-7.
22. Martial A, Dardenne M, Engasser JM, Marc A. Influence of the age on hybridoma culture kinetics. Cytotechnol 1991;5:165-71.
23. Jackson L, Trudel L, Lipman N. Small-scale monoclonal antibody production in vitro: methods and resources. Lab Animal 1999;28:38-50.
24. Marx U, Embleton J, Fisher R, Gruber F, Hansson U, Heuer J, et al. The report and recommendations of ECVAM workshop. ATLA 1997;23:121-37.
Received in December, 2008.
Accepted for publication in June, 2009.
Rodolfo Valdés. Monoclonal Antibody Department, Center for Genetic Engineering and Biotechnology, CIGB Ave. 31 / 158 and 190, Cuabanacán, Playa, PO Box 6162, CP 10600, Havana, Cuba. E-mail: rodolfo.valdes@cigb.edu.cu