My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.3 La Habana July-Sept. 2009
REPORT
GM3 ganglioside: a novel target for the therapy against melanoma *
Zaima Mazorra1, Cirse Mesa2, Luis E Fernández2
1Department of Clinical Immunology
2Department of Vaccines
Center of Molecular Immunology, CIM Ave. 216, corner 15, Atabey, Playa, PO Box 16 040, Havana, Cuba
ABSTRACT
Malignant melanoma is a tumor with a steeply increasing incidence and scarce therapeutic options once metastatic. Currently, no vaccine is widely commercially available for melanoma treatment or prevention. The overexpression of GM3 ganglioside in murine and human melanomas and its important role in tumor progression makes this self antigen a potential target for preventive immunotherapy of this neoplasm. Previously, we have shown that preventive vaccination with GM3/VSSP induced a specific antitumor response; and elicited the rejection of syngeneic GM3- positive melanoma cells in immunized mice. In the present paper, we published the induction of a potent antitumor effect of this vaccine administered in a minimal residual disease B16 melanoma model. These findings propose the GM3/VSSP vaccine as a therapy designed to elicit and/or boost antitumor immunity in patients with minimal residual disease after surgery; thereby preventing or prolonging the time to recurrence. This is an important issue of the clinical setting because patients with stage II melanoma were reported to have 60% chance of survival 5 years after surgery. In addition, we examined the mechanisms by which this immunogen confers tumor protection. Surprisingly, in spite of the glycolipidic nature of this antigen, we have found that induction of anti-GM3 IgG antibodies and tumor- specific IFN γ secreting CD8+ T cells correlated with tumor protection. As a result, these findings demonstrate, for the first time, the direct involvement of the cellular immune response in the anti-tumor protection induced by a ganglioside-based vaccine.
INTRODUCTION
The incidence of malignant melanoma is rising faster than any other malignancy. A large number of patients once develop metastatic disease, lack of an effective therapeutic option (1). In the therapeutic cancer vaccines field, the largest accumulated clinical experience, so far, has been in advanced stage melanoma, though with rather modest outcomes in most of the clinical studies (2). One of the reasons for this poor effectiveness is that the antitumor vaccination trials have included patients in late stages of the disease in which the development of tumor-induced immunosuppression significantly interferes with immunization. It is likely that active immunotherapy could operate more efficiently early during tumor progression or could prevent the disease in patients at a high risk of having malignant lesions. For these reasons, the therapeutic success of a GM3-based vaccine in patients with earlystage melanoma remains an interesting and open question, but more preclinical evidence in a relevant animal model is needed.
Gangliosides are a family of sialic acid-containing glycosphingolipids frequently over expressed in the external membrane of cancer cells, not only of neuroectodermal origin. Ganglioside vaccines have been clinically tested in different types of advanced cancers, mainly melanomas (3), based on the observation that altered expression of these glycolipids in melanoma cells correlated with their metastatic potential. Additionally, GM3 and GD3, the major gangliosides in melanomas, are shed into the tumor microenvironment which can promote severe immune dysfunctions (4, 5). It has been claimed that the induction of anti-GM3 antibodies circumvents this specific immunosuppression (5, 6). The role of antibodies in the anti-tumor effect of ganglioside vaccines has been considered to be predominant, if not exclusive (7).
However, the involvement of T cells in the antitumor protection induced by ganglioside-based vaccines has not been described yet. This is an important issue, since if a robust cellular immune response could be elicited, then immunological memory could be long lasting, eradicating tumor before it becomes clinically obvious.
In the present work, we described the induction of a potent anti-tumor effect of the GM3/VSSP vaccine in combination with surgical excision of the primary tumor burden. In addition, we examined the mechanisms by which this immunogen confers tumor protection.
RESULTS AND DISCUSSION
In order to find new effective therapies against melanoma, a novel vaccine was tested in the B16 mouse melanoma model. This vaccine is composed of very small sized proteoliposomes (VSSP) resulting from the hydrophobic incorporation with GM3/VSSP ganglioside into Neisseria meningitidis natural outer membrane vesicles (8). We have previously shown experimental data indicating that preventive immunization of mice with the GM3/VSSP vaccine elicited the rejection of B16 melanoma tumors (6). However, the capacity of this vaccine to generate long lasting protection has not been studied. The duration of antitumor protection after pre-immunization with four biweekly i.m. doses of 120 μg GM3/VSSP by injecting a low tumor burden of 2.5 x 103 B16F0 cells subcutaneously (S.C.) was investigated. In different sets of experiments, at least 80% of control animals developed s.c. tumors and died 46.5 ± 5.7 days (mean ± SE) after challenge. Complete antitumor protection was obtained from 21 to 35 days after the administration of the last dose of the vaccine. A significant antitumor protection was still maintained 91 days after tumor inoculation.
The precise mechanisms by which this vaccine confers tumor protection were unknown. Here we reported the cellular and humoral requirements for the antitumor activity of VSSP/GM3 vaccine in the prophylactic B16 melanoma model. The role in melanoma protection of the anti-ganglioside antibody response, detected in vaccinated mice, was addressed by injecting C57BL/6 mice with four doses of VSSP/GM3 + Montanide ISA 51. Mice were challenged with 104 B16 melanoma cells on day 63. Sera were collected four times until day 93 and the anti-GM3 IgG and IgM titers were measured by ELISA. Before immunization, antibodies against GM3 were absent from animal sera. Curiously, in protected mice, median anti-ganglioside IgG titers were 1:640, 1:480 and 1:240 in days 56, 70 and 93, respectively, while in unprotected mice these values were 1:120, 0 and 0 at the same time points (p < 0.05, Mann Whitney test) (Figure 1). Interestingly, in protected mice, specific IgG serum antibodies persisted, in almost all animals, at least 50 days after the last vaccination while median IgM titers remained similar irrespective of the vaccine outcome. Noteworthy, a significant direct correlation between the magnitude of the vaccine induced anti-GM3 IgG antibody response and the tumor rejection capacity was observed in each post-immunization time point (p < 0.05, Spearman correlation).
In line with these findings, our clinical results suggest a positive correlation between antiganglioside IgG antibodies titers and prolonged survival of advanced breast cancer patients immunized with a gangliosidebased vaccine (manuscript in preparation). In contrast, previous studies have shown an association between vaccine-induced anti-GM3 IgM antibodies and tumor protection when C57BL/6 mice were immunized with a melanoma cell-based vaccine (9). These results have been also found in clinical studies, in which a positive correlation between elevated antiganglioside IgM antibody titers and improved survival for melanoma and soft tissue sarcoma patients has been reported. It has been speculated that anti-ganglioside IgM antibodies are effective against tumors because the capacity of these immunoglobulins to neutralize and clear immunosuppressive gangliosides, eliminating tumor cells by complement mediated cytotoxicity (CMC) (10). Regarding the functional properties of the anti-GM3 IgG antibodies induced by the VSSP/GM3 vaccine, we previously reported their capacity to evoke CMC against B16F10 cells in BALB/c mice using heterologous complement (6). In the present work, we found that anti-GM3 antibodies that persisted after tumor challenge in surviving-vaccinated mice were able to induce CMC. In this case, autologous complement was used. However, this result should be confirmed in further experiments with larger amount of mice, the CMC activity by IgG antibodies may play an important role in melanoma eradication.
Despite the crucial role of cellular immune response in tumor protection, the involvement of T cells in anti-tumor immunity of ganglioside vaccines was not described before. This is an important issue, since if a robust cellular immune response could be elicited, the immunological memory could be long lasting, eradicating tumor before it becomes clinically obvious. In our in vivo depletion experiments, we found that CD8+ T cells were essential for tumor protection (Figure 2A). Immunized mice lacking effectors CD8+ T cells were unprotected from tumor challenge if compared with their competent littermates (p > 0.05, Log-rank test). Moreover, T cells from surviving-immunized mice were able to secrete significant amounts of IFNγ only if they were co-cultured with MHC class I high B16 melanoma cells (p < 0.05, Mann Whitney test). When the stimulus came from 3LL-D122 cells (also pre-treated with IFNα) IFNγ was almost absent from the supernatants (Figure 2B). T cells did not release IFNγ in the presence of MHC I low counterparts cell lines (data not shown). It is known that B16 melanoma is a tumor with well-established mechanisms of tolerance induction and immune escape. One of these mechanism is the down regulation of class I MHC molecules and antigen processing machinery (11). Moreover, it has been extensively shown that IFNγ is required in vaccine-induced antitumor immunity; it is specifically effective against B16 melanoma cells (12). Considering these findings, we could hypothesize that the vaccine could induce cytokine release such as interferons able to up-regulate the MHC I expression on tumor cells, which could be then eliminated by CTL specific for melanoma antigens. By contrasting, up-regulation of MHC I expression prevents recognition of tumor cells by NK cells which, in our data set, were not necessary on the effect or phase of the antitumor response. These results indicate how the vaccination process induced, in protected mice, effectors CD8+ T cells specific for melanoma antigens.
It is known that mice with experimental tumors, as well as patients with cancer, show a decreased immunologic potency. This tumor induced immunosuppression is reinforced in patients with high tumor burdens (13). Consequently, in the last few years, our view of the range of applications of tumor vaccines has changed because treatment of patients with minimal residual disease or with low staging showed better responses to vaccination. Furthermore, an open question remains regarding whether vaccines have sufficient benefit for exploration in adjuvant or even in preventive conditions. The answers to these questions could be found, in part, by exploring the responses observed with the correct use of animal models.
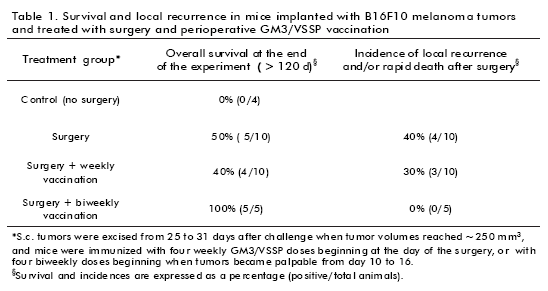
Taking into consideration these findings, we have explored immunization with GM3/VSSP vaccine in combination with surgical excision of the primary tumor mass. The results showed that this model could clear the response to vaccination in combination with resection of the highly aggressive B16F10 melanoma. In this scenario, we observed that mice subjected to surgery plus a perioperative vaccination protocol consisting of four GM3/VSSP doses every 14 days beginning before surgery have an increased survival period compared with any other group. Furthermore, all mice showed neither local recurrence nor visible lung metastasis at the end of the experiment (Table 1). Interestingly, mice under a similar perioperative immunization with four doses of the vaccine, but administered every 7 days, showed reduced survival, similar to animals treated with surgery alone.
Because several clinical protocols involve active immunotherapy in cancer patients with large primary tumors or post-surgery patients, the functional effect of the tumor mass in the immune response would be an important feature. In this sense, we studied the functional immunologic variables following immunization with a foreign antigen, such as ovoalbumin (OVA) in tumor-bearing and healthy mice. The purpose of the experiment was to examine the response to vaccination with OVA before and after tumor surgery and the possible kinetics of immune recovery after excision of the tumor mass. In immunocompetent mice, this antigen should induce a strong and specific response. For this purpose, OVA-specific DTH was assayed by immunizing two doses of OVA in complete Freunds adjuvant. Compared with tumor-bearing mice, CD4+ T-cell response in B16F10-operated mice showed a significant recovery after surgery, measured by ovoalbumin antigen-specific DTH (p < 0.05, Tukey test). However, this response is largely distant from the DTH developed in healthy, sham-operated mice (p < 0.01, Tukey test). To analyze whether surgical excision of primary tumor recovers CD4+ T-cell function, B16F10-operated mice were immunized at 2, 15, and 30 days after surgery with OVA emulsified in complete Freunds adjuvant. Seven days later, inguinal lymph nodes were removed, cells were cultured for 4 days with OVA, and the supernatant was collected for cytokine quantification by ELISA. Strikingly, immunization induced a strong IL-4 secretion in B16F10-operated mice as compared with healthy animals (p < 0.05, Students t test), but values returned to normal 30 days after tumor excision. Few studies have indicated that immune system functions in cancer patients recover after surgery (14). However, there are no precedent studies of this phenomenon for melanoma. Our results showed that the B16F10 tumor could induce a CD4+ T-cell dysfunction and not rapidly overcome, lasting for at least 30 days after surgical treatment. In this scenario, four biweekly immunizations with GM3/ VSSP during the immunosuppression window induced a strong antitumor response. This fact suggests that our vaccine, given in a correct immunization protocol, could be able to increase the immune responsiveness and allow the immune system to prevent tumor recurrence or metastasis.
All these preclinical data strongly suggest a combined therapeutic effect of tumor excision and vaccination with GM3/VSSP every 14 days. This is an important issue to translate to the clinical setting because patients with stage II melanoma were reported to have a 60% chance of survival five years after surgery. Some patients with stage II melanoma are at high risk for recurrent disease, and occult micro metastases cause recurrence following treatment with surgery alone (15). According to the results obtained with the present preclinical model, vaccination with GM3/ VSSP could significantly increase survival after the surgical management of primary cutaneous melanoma.
CONCLUDING REMARKS
Altogether, these preclinical experiments propose the GM3/VSSP vaccine as a therapy designed to elicit and/or boost antitumor immunity in patients with minimal residual disease after surgery, thereby preventing or prolonging the time to recurrence. Future clinical trials will be needed for a definitive confirmation about the beneficial role of the GM3/VSSP vaccine in the perioperative handling of patients with stage II melanoma. Regarding to the mechanism by which GM3/VSSP confers, for the first time, it was demonstrated the direct involvement of the cellular immune response in the antitumor protection induced by a ganglioside-based vaccine.
ACKNOWLEDGEMENTS
The authors thank Audry Fernández, Dr. Mariano R Gabri, Dr. Giselle V Ripoll and Dr. Daniel E Gómez for their important contribution to this work. In addition, we would like to thank Dr. Carmen Viada for her statistical advice and Armando López for his excellent technical assistance.
REFERENCES
1. Koon HB, Atkins MB. Update on therapy for melanoma: opportunities for patient selection and overcoming tumor resistance. Expert Rev Anticancer Ther 2007;7:79-88.
2. Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med 2004;10:909-915.
3. Kananath D, Hill AD, Djikstra B, Kenelly R, McDermott EM, OHiggins NJ. Adjuvant therapies in the treatment of stage II and III malignant melanoma. Surgeon 2005;3:245-56.
4. Kudo D, Rayman P, Horton C, et al. Gangliosides expressed by the renal cell carcinoma cell line KK-RC-45 are involved in tumor-induced apoptosis of T cells. Cancer Res 2003;63:1676-83.
5. Dohi T, Nores G, Hakomori S. An IgG3 monoclonal antibody established after immunization with GM3 lactone: immunochemical specificity and inhibition of melanoma cell growth in vitro and in vivo. Cancer Res 1988;48:5680-5.
6. Carr A, Mazorra Z, Alonso DF, et al. A purified GM3 ganglioside conjugated vaccine induces speciWc, adjuvant-dependent and non-transient antitumor activity against B16 mouse melanoma in vitro and in vivo. Melanoma Res 2001;11:219-27.
7. Livingston PO, Ragupati G. Carbohydrate vaccines that induce antibodies against cancer. 2. Previous experience and future plans. Cancer Immunol Immunother 1997;45:10-9.
8. Estévez F, Carr A, Solorzano L, et al. Enhancement of the immune response to poorly immunogenic gangliosides after incorporation into very small size proteoliposomes (VSSP).Vaccine 2000;18:190-7.
9. Ravindranath MH, Brazeau SM, Morton DL. Efficacy of tumor cell vaccine after incorporating monophosphoryl lipid A (MPL) in tumor cell membranes containing tumor-associated ganglioside. Experientia 1994;50:648-53.
10. Ragupathi G, Liu NX, Musselli C, Powell S, Lloyd K, Livingston PO. Antibodies against tumor cell glycolipids and proteins, but not mucins, mediate complement-dependent cytotoxicity. J Immunol 2005;174:5706-12.
11. Lizee G, Radvanyi LG, Overwijk WW, Hwu P. Immunosuppression in melanoma immunotherapy: potential opportunities for intervention. Clin Cancer Res 2006;12:2359s-65s.
12. Winter H, Hu HM, McClain K, Urba WJ, Fox BA. Immunotherapy of melanoma: a dichotomy in the requirement for IFN g in vaccine-induced antitumor immunity versus adoptive immunotherapy. J Immunol 2000;166:7370-80.
13. Kiessling R, Wasserman K, Horiguchi S. Tumor induced immune dysfunction. Cancer Immunol Immunother 1999;48:353-62.
14. Barbieri C, Fujisawa MM, Yasuda CL, et al. Effect of surgical treatment on the cellular immune response of gastric cancer patients. Braz J Med Biol Res 2003;36:339-45.
15. Ross MI. Early-stage melanoma: staging criteria and prognostic modeling. Clin Cancer Res 2006;12:312-9.
*This work received the Award of the National Academy of Sciences of Cuba for the year 2008.
Zaima Mazorra. Department of Clinical Immunology. Center of Molecular Immunology, CIM. Ave. 216, corner 15, Atabey, Playa, PO Box 16 040, Havana, Cuba. E-mail: mailto:zaima@cim.sld.cu