My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.26 no.4 La Habana Oct.-Dec. 2009
REPORT
HER1 Vaccine: An autologous EGFR vaccine candidate to treat epithelial tumors
Belinda Sánchez, Yeranddy Aguiar, Diana R Hernández, Greta Garrido, Rolando Pérez, Luis E Fernández
Center of Molecular Immunology, CIM 216 corner 15, PO Box 11 600, Atabey, Playa, Havana, Cuba
ABSTRACT
The Epidermal Growth Factor Receptor (EGFR) plays an essential role in regulating neoplastic processes. EGFR over-expression in many human epithelial tumors has been correlated with disease progression and bad prognosis. Passive EGFR-directed immunotherapy has been introduced in medical oncology practice, but no active specific approaches have been used. We designed a vaccine candidate based on the extracellular domain (ECD) of human EGFR (HER1), and the homologous vaccine for mice based on murine EGFR. This vaccine candidate uses the Very Small-Sized Proteoliposomes from Neisseria meningitidis (VSSP) and Montanide ISA 51-VG as adjuvants. The autologous vaccine circumvents the tolerance to self EGFR by inducing a specific immune response with an anti-metastatic effect on EGFR+ tumors. The vaccine candidate based on HER1-ECD induced anti-EGFR polyclonal antibodies (PAb) that bind EGFR+ human tumor cell lines from different tissues. These anti-EGFR PAb abrogate ligand-dependent EGFR phosphorylation, provoking the inhibition of tumor cell growth and apoptosis. Preclinical results further encouraged the evaluation of the HER1 vaccine candidate in phase I clinical trials.
INTRODUCTION
The human epidermal growth factor receptor (EGFR or HER1) belongs to the erbB family of four closely related cell membrane receptors, also known as the Type I Receptor Tyrosine Kinase family: HER1/erbB1, HER2/erbB2, HER3/erbB3 and HER4/erbB4 (1, 2). The four receptors consist of an extracellular ligand-binding domain (ECD), a transmembrane domain, and an intracellular domain with tyrosine kinase activity for signal transduction. EGFR plays an essential role in regulating both development and neoplastic processes. On binding their specific ligands, such as the epidermal growth factor (EGF) or transforming growth factor alpha (TGF-α), among others, there is an induction of receptor activation, modulation of cell proliferation and the differentiation in normal tissues and tumors. EGFR can be found over-expressed or mutated in many human epithelial tumors such as those of breast, lung, prostate, head and neck, colorectal, pancreatic, bladder, vulva and ovarian tumors. This over-expression has been correlated with disease progression and poor prognosis (3). The activation of the EGFR signaling pathway in cancer cells has been shown to enhance cell proliferation, angiogenesis, tumor promotion and metastasis, and to decrease apoptosis. The potential of EGFR-targeted therapies for cancer treatment has increased the development of different passive agents (4), but active specific approaches have not been introduced in medical oncology practice. HER1-based active specific immunotherapy may be an alternative and complementary approach to treat epithelial tumors, provided the induction of an immune response against self EGFR is feasible.
RESULTS AND DISCUSSION
In order to design a vaccine based on Her1-ECD, the cDNAs encoding both Her1-ECD and murine EGFR-ECD (mEGFR-ECD) were successfully cloned into the pcDNA3 expression vector and used to transfect HEK293 cells (5). The identity and subsequent potential of recombinant proteins such as immunogens were demonstrated through their recognition by specific MAbs. The vaccine candidate included VSSP (very small size proteoliposomes) a new product that has been clinically tested in humans, and Montanide ISA 51 (Mont) as adjuvants. VSSP was selected because of its properties in maturing dendritic cells by inducing IL-12 production, which in turn is vital for pro-inflammatory responses (6).
The humoral response of the autologous vaccine was explored by immunizing C57BL/6 mice 4 times biweekly with 50 μg of mEGFR-ECD in VSSP/ Mont. Inoculated mice developed specific cellular and humoral immune responses. The high serum IgG antibody levels comprised IgG2a, IgG2b and IgG1 subclasses, which increased with successive immunizations. It was thus demonstrated that vaccination breaks the tolerance to EGFR, which constitutes a central deletion antigen expressed in the thymus (5). The high immunogenicity of the antigen could be derived from the presentation context and the use of a truncated protein that could modify the immunodominance of the T cell repertoire. To check whether immunizations with a truncated EGFR affects the recognition of the full length EGFR in its native conformation on the cell surface, EGFR+ cells were analyzed by FACS. EAT and 3LL-D122 murine cell lines were positively stained by mEGFR-ECD/ VSSP/ Mont-immunized mice sera. These sera showed in vitro anti-tumor effect, inhibiting EGFR+ tumor cell proliferation and decreasing the number of viable cells (5).
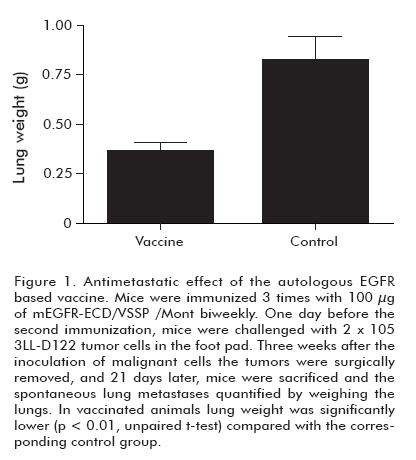
To investigate whether autologous vaccination can protect individuals from widespread metastases, mice received a first immunization with the mEGFR-ECD in VSSP/ Mont and, 1 day before the booster immunization, they were challenged with 2 x 105 tumor cells in the foot pad. Three weeks after inoculating malignant cells, tumors were surgically removed. Twenty-one days after surgery, mice were sacrificed and the spontaneous lung metastases were quantified by weighing the lungs. As shown in Figure 1, vaccination significantly reduced lung metastasis of mice (p < 0.01), compared to the control group (5). The anti-metastatic model we have used simulates the medical practices in which physicians remove the primary tumors and sometimes, depending on the stage of the tumor, metastatic disse-mination can occur. In fact metastatic dissemination is normally responsible for the death of the patients. That is the reason why avoiding metastases is the main challenge for cancer vaccines. The anti-metastatic effect of the HER1 vaccine involved the cellular immune response, which was demonstrated by depletion experiments (unpublished data). The specificity of these CD8+ T cells is currently studied. On the other hand, potential side effects of “self”’ immunization in humans were stressed by examining the appearance of reproductive anomalies in female mice immunized with the mEGFR-ECD in VSSP/Mont. After vaccination, animals were mated and their progeny studied. Pregnancy rates, pups per litter, birth weights, hair growth, eyes opening and incisor appearance parameters did not differ between the vaccinated and the control group of mice (Mann Whitney U test, p > 0.05). Besides, a group of mice was observed for 1 year after the end of the immunization protocol, and vitality, temperature and food intake were completely normal No changes were found in functional hepatic parameters when compared to those of non-immunized mice (5).
Once the proof of principle of autologous vaccination based on mEGFR, was demonstrated we characterized the immune response, in mice, induced by the vaccine candidate intended for clinical studies in EGFR+ epithelial tumor patients: HER1-ECD/ VSSP/Mont. This vaccine activated the cellular immune response in mice, which was demonstrated by the induction of specific DTH response and the stimulation of T lymphocytes (7). Furthermore, immunization induced high specific IgG antibody titers, which increased by increasing the number of HER1-ECD doses administered in the vaccine formulation (8). Curiously, polyclonal antibodies (PAb) generated by HER1-ECD vaccination were poorly cross-reactive with mEGFR-ECD in spite of the high homology in primary sequence (87%) between these two proteins (5). This result supports the concept of autologous vaccination, indicating that the patient-directed vaccine should contain the human EGFR-ECD protein. Vaccination-induced PAb recognized a panel of EGFR+ human tumor cell lines of different origins and levels of receptor expression, such as vulva, lung, colon, breast and glioma (Figure 2a) (8). This result suggests that the HER1 vaccine could be used in a wide array of patients with different EGFR+ tumor origins, irrespective of the level of EGFR expression. It was also demonstrated that these PAb are capable of inhibiting EGF and TGFα binding to EGFR+ tumor cells (Figure 2b). This could abrogate the ligand-dependent EGFR phosphorylation. It inhibits the activation of signaling cascade proteins, such as Erk1/2, which are directly connected to cell proliferation. In fact, the incubation with the specific vaccine PAb arrested EGFR+ tumor cell lines at the G0/G1 phase, it inhibited cell proliferation (Figure 2c) and induced apoptosis (8).
We conclude that self EGFR-ECD is immunogenic in VSSP contexts, specifically activating the humoral and cellular immune responses. The anti-metastatic effect of the vaccine involves CD8+ T cells, suggesting the activation of cytotoxic T lymphocytes. The antibodies produced by vaccination recognize a wide array of EGFR+ tumor cells and shows anti-proliferative and anti-apoptotic effects as a consequence of ligand-dependent EGFR inhibition. As a whole, these results indicate that the HER1 vaccine can be an attractive therapeutic approach to treat patients with EGFR+ tumors.
REFERENCES
1. Ullrich A, Coussens L, Hayflick JS, Dull TJ, Gray A, Tam AW, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature 1984;309(5967):418-25.
2. Normanno N, Bianco C, Strizzi L, Mancino M, Maiello MR, De Luca A, et al. The ErbB receptors and their ligands in cancer: an overview. Curr Drug Targets 2005;6(3):243-57.
3. Khademi B, Shirazi FM, Vasei M, Doroudchi M, Gandomi B, Modjtahedi H, et al. The expression of p53, c-erbB-1 and c-erbB-2 molecules and their correlation with prognostic markers in patients with head and neck tumors. Cancer Lett 2002;184(2):223-30.
4. Crombet T, Torres L, Neninger E, Catala M, Solano ME, Perera A, et al. Pharacological evaluation of humanized anti-epidermal growth factor receptor, monoclonal an-tibody h-R3, in patients with advanced epithelial-derived cancer. J Immunother 2003;26(2):139-48.
5. Ramírez BS, Pestana ES, Hidalgo GG, García TH, Rodríguez RP, Ullrich A, et al. Active antimetastatic immunotherapy in Lewis lung carcinoma with self EGFR extracellular domain protein in VSSP adjuvant. Int J Cancer 2006;119(9):2190-9.
6. Mesa C, De Leon J, Rigley K, Fernández LE. Very small size proteoliposomes derived from Neisseria meningitidis: an effective adjuvant for Th1 induction and dendritic cell activation. Vaccine 2004;22(23-24):3045-52.
7. Ramírez BS, Alpizar YA, Hidalgo GG, Capote AR, Fernández DR, Rodríguez RP, et al. Specific immune response induced in mice by immunization with the human Epidermal Growth Factor Receptor extra-cellular domain. Biotecnol Apl 2008;25: 135-40.
8. Ramírez BS, Alpizar YA, Fernández DR, Hidalgo GG, Capote AR, Rodríguez RP, et al. Anti-EGFR activation, anti-proliferative and pro-apoptotic effects of polyclonal antibodies induced by EGFR-based cancer vaccine. Vaccine 2008;26(38):4918-26.
Belinda Sánchez. Center of Molecular Immunology, CIM 216 corner 15, PO Box 11 600, Atabey, Playa, Havana, Cuba. E-mail: belinda@cim.sld.cu