My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.27 no.4 La Habana Oct.-Dec. 2010
TECHNIQUE
Influence of sample quality on phenylalanine and 17-hydroxyprogesterone levels in neonatal screening
Calidad de la muestra y niveles de fenilalanina y 17-hidroxiprogesterona en pesquisa neonatal
Lesley del Río Fabre, Ernesto C González, Amarilys Frómeta, Elisa M Castells, Yileidis Tejeda
Laboratorio de Pesquisa Neonatal, Centro de Inmunoensayo, CI Calle 134 y Ave. 25, AP 6653, Cubanacán, Playa, Ciudad de La Habana, Cuba
ABSTRACT
Quality of the sample and phenylalanine and 17-hydroxyprogesterone levels in neonatal screening. In the programs for neonatal screening many different analytes are quantified from dried blood on filter paper cards. Several factors affect the quality of the samples invalidating their employment in the laboratory: inadequate collection procedures, quality of the filter paper, the drying, storage and transportation under extreme environmental conditions. This article aims to show how the quality of the sample collection influences the phenylalanine (Phe) and 17-hydroxyprogesterone (17-OHP) levels. As such, samples of newborns on filter paper from Ramon Gonzalez Coro Hospital were collected and analyzed.The blood spots from a sample were classified according to their appearance, satisfactory spot (MV) or unsatisfactory spot (MNV). Phe and 17OHP levels were determinated by UMTEST PKU and UMELISA 17OH Progesterone NEONATAL, respectively. 19.5% of 3043 newborns samples exhibited unsatisfactory spots. Concordance correlations studies between MV y MNV showed the precision and accuracy were affected by quality of the samples. False positive and negative values (with concerning control sample) were detected from samples with MNV. The correct application of collection procedures for samples is essential in obtaining reliable results in screening neonatal laboratories.
Keywords: dried blood spots, filter paper, neonatal screening, quality, phe, 17-OHP.
RESUMEN
En los programas de pesquisa neonatal, diferentes analitos son cuantificados en muestras de sangre de recién nacidos, impregnadas sobre papel de filtro. Varios factores pueden afectar la calidad de las muestras e invalidar su empleo en el laboratorio: procedimientos inadecuados en la colecta, calidad del papel de filtro y procesos de secado, almacenamiento y transportación bajo condiciones ambientales extremas. Para documentar la influencia de la calidad de la muestra sobre los niveles de fenilalanina y 17-hidroxiprogesterona en manchas de sangre seca sobre papel de filtro, se analizaron muestras de recién nacidos provenientes del Hospital Materno-Infantil "Ramón González Coro". Las manchas provenientes de una misma muestra se clasificaron según su apariencia, en válidas o "no válidas". Los niveles de fenilalanina y 17- hidroxiprogesterona se determinaron mediante el empleo de UMTEST PKU y UMELISA 17OH Progesterona NEONATAL, respectivamente. De 3 043 muestras analizadas, el 19.5% presentó manchas con características "no válidas". Los estudios de concordancia de la correlación entre ellas, mostraron que la calidad de la muestra afectó la precisión y la exactitud de los niveles de fenilalanina y 17-hidroxiprogesterona en sangre seca. En las muestras con características "no válidas" fueron detectados resultados discordantes, con valores falsos positivos o negativos con respecto a la muestra control. La aplicación correcta de los procedimientos de colecta de las muestras de pesquisa neonatal es esencial para la obtención de resultados confiables en los laboratorios.
Palabras clave: sangre seca, papel de filtro, pesquisa neonatal, calidad, fenilalanina, 17-hidroxiprogesterona.
INTRODUCTION
Inborn metabolic disorders comprise a wide set of diseases commonly appearing at pediatric ages. The lack of early diagnosis may lead to patient death, or bring about severe neurological sequelae with a pessimistic prognosis for learning and social behavior (1).
Neonatal screening (NS) of inherited metabolic disorders is an essentially preventive procedure aimed at detecting and unequivocally identifying a condition which could potentially lead to a serious illness (2). Therefore, NS is applied to apparently healthy newborns before symptoms of the disease develop, which allows start treatment appropriately prior to irreversible effects.
Blood collected on filter paper cards is the routine method used for sampling due to its easy transportation from distant places to the central laboratory for testing, not needing refrigeration or special care (3). Besides, samples can be properly stored and preserved by using this procedure, without decreasing their stability and facilitating laboratory processing and handling. This method for sample collection implies a minor biological risk and requires a very small amount of blood (4).
However, several factors may affect sample quality and invalidate its laboratory processing like: inadequate sampling procedures, filter paper card quality and the drying process, storage and transportation under extreme environmental conditions (4, 5), among others. Samples can be classified into satisfactory or unsatisfactory depending on the compliance or not with these procedures. A sample whose blood spot is approximately a centimeter in diameter is regarded as valid and distributed homogeneously in both paper sides, enough to perform various 3mm cuts at its central zone (6). In general, the unsatisfactory or invalid specimens is inappropriately taken, handled or transported to the laboratory, showing scratches, inadequate color, visible contamination or incomplete identification data. Invalid samples can be classified according to their appearance in:
• Insufficient sample (IS): A sample having insufficient blood amount, caused by removing the filter paper card before being absorbed to the opposite side.
• Scratched or abraded sample (SS): A sample presenting scratches or appearing wearied due to applying blood with a capillary tube or another device.
• Supersaturated sample (SSS): A sample showing stains of excessive dry blood on the filter paper card, probably caused by the device used or applying blood in both sides of the card.
• Apparently diluted, faded or contaminated sample (CS): probably before or after being obtained, filter paper got in contact with the hands or with substances like alcohol, antiseptic solutions, water, powder or others. Sample could be exposed to heat or the punction area also be excessively compressed.
• Sample with serum rings (RS). These rings are spotted due to alcohol was not thoroughly removed from the punction area at the heel or the area resulted excessively compressed.
• Clot-bearing Sample (CBS): The same paper circle got in contact with blood drops more than once.
Phenylketonuria (PKU) is an inherited autosomal recessive disorder affecting phenylalanine (Phe) catabolism. This disorder is produced by a deficiency of the phenylalanine-hydroxylase enzymatic complex which catalyzes Phe conversion in tyrosine (7). Such complex originates the accumulation of Phe in blood, in other liquids and in organic tissues, as well as a slight diminishing in tyrosine levels in blood. Phe concentration in the newborn carrier may be normal till the fourth day of living, but rapidly augments while protein feeding starts (7). If treatment at the newborn period is not applied, clinical signals of the disease, -among which: irreversible mental retardation, neurological abnormalities and skin defects and its pigmentation-, appear. Hence, early diagnostics and the application of a diet Phe steadily scarce, with enough quantity of such aminoacid for the normal growth of the child are highly important (8, 9).
Congenital adrenal hyperplasia, the most common of genital ambiguity is caused by the deficiency of one of the five enzymes involved in the biosynthesis of corticosteroids at the adrenal cortex and leading to different manifestations of the disease, in which in 90- 95% of cases the cause is deficiency of 21-hydroxilase enzyme (10). Such deficiency is originated by the accumulation of high levels of 17a-hydroxiprogesterone (17-OHP), main biochemical marker for the detection of the disease (10). The common feature of these defects is the presence of low levels of cortisol with the consequent hyperplasia of the adrenal cortex, due to the hyper-secretion of corticotropine at the hypophysis. Among associated symptoms to the disease severe dehydration, feminine external genitalia virilization and the premature development of secondary male sexual characteristics are found, preventable with the early diagnostics of the disease and the beginning of a replacement therapy with steroids (11).
Documenting the influence of sample quality upon phenylalanine levels (Phe) and 17-hydroxiprogesterone (17-OHP) in newborn dry blood stains on filter paper used at the NS programs was the objective of this work.
MATERIALS AND METHODS
Samples of 3043 newborns on filter paper from "Ramón González Coro" Hospital were collected and analyzed. Samples were classified according to simultaneous presence of satisfactory or valid spots (VS) and unsatisfactory or invalid spots (IVS) and were evaluated in duplicate. Phe and 17-OHP levels were determined by the use of the UMTEST PKU and UMELISA 17OH Progesterone Neonatal, respectively. Both kits were produced by the Immunoassay Center, Havana, Cuba (12, 13).
SUMA® technology equipment was used for quantification, validation and interpretation of results: manual punch device P-51, automated plate washer MAS 301, fluorometer-photometer reader PR-521 and the Strip Reader software (Version 9).
Phe levels were quantified by UMTEST PKU, a fluorescent test based on the McCaman & Robins method (12). For the measurement of Phe concentrations, 3 mm blood discs of calibrators, control and samples were punched out into each well of the elution microplates, and incubated with 70 ml ethanol 70% (v/v) in a humid chamber for 30 min at 20 -25 °C. Afterwards, 10 mL of eluate was transferred to white opaque polystyrene ultramicroplates containing 10 ml of reaction mixture (ninhydrin and L-leucyl-L-alanine).
Plates were incubated at 60 °C for 1 h in a humid chamber. A fluorescent complex was obtained by adding 10 ml of copper reagent to the reaction ultramicroplate, and 5 - 15 min; later, fluorescence was automatically measured in the fluorometer-photometer reader.
17OHP levels were determined by using UMELISA 17OH Progesterone Neonatal kit, a simple and rapid competitive ultramicro ELISA assay based on competition between 17-OHP alkaline phosphatase conjugate and 17-OHP in blood specimens for a limited number of binding sites on specific polyclonal rabbit anti-17-OHP antibodies (13). For the measurement of 17-OHP concentrations, 3 mm blood discs of standards, controls and samples were punched out of the filter paper and placed into each well of the elution microplates, followed by the addition of 40 ml of the diluted 17-OHP-alkaline phosphatase conjugate. After the elution in a humid chamber for 30 min, at 20-25 °C, 10 ml of eluate were transferred into the well of the reaction´s opaque polystyrene ultramicroplates, coated with the specific polyclonal rabbit anti-17-OHP antibodies. The competitive reaction occurred for 2 hrs at 20-25 °C in a humid chamber and then, the plates were washed 6 times by adding 28 mL of washing solution using the automated plate washer MAS 301. The fluorogenic reaction was performed by adding 10 ml of 4-methylumberipherylphosphate substrate. The ultramicroplates remained at 20-25 °C in a humid chamber for 30 min. Finally, the fluorescence was measured automatically in the fluorimeter-photometer reader. Automatic validation and interpretation of the results were done using specific-assay software.
Statistical analyses
Data were stored and processed through a Microsoft Excel 2007 spread sheet. Phe concentration mean values and 17-OHP from VS and IVS were calculated. We assessed the quantitative differences between VS and IVS using 2 methods: the Pearson correlation and the concordance correlation coefficient (rc). Statistical parameters, r12 and c12, were calculated as a measure of precision and accuracy indicators between these variables, respectively (14). The concordance correlation coefficient was calculated as a measure of agreement between the analytes levels from VS and IVS. Values 0.2 - 0.7 indicate minor concordance; values 0.7 - 0.85 indicate moderate concordance, and values higher than 0.85 indicate clear to high concordance (14). The slope, y-intercept of the line-ofbest- fit, and regression coefficient were calculated. Groups were compared by the Mann-Whitney U test with a probability of 95% using the Statgraphics, version 5.1.
RESULTS AND DISCUSSION
From the 3 043 newborn samples analyzed, 19.5% (592) showed stains with certain invalid characteristics. Phe concentrations and 17-OHP in each IVS and VS from a same sample were determined. The stain complying with the standard requisites established by the guidelines issued by the National Committee for Clinical Laboratory Standards of the United States (NCCLS) was considered as control spot (6). Phe mean concentration value for both groups was determined. The mean value obtained for IVS was 87.7 mmol Phe/L whole blood, being slightly higher than the total of VS, 82.4 mmol/L.
Correlation study and the equation of the straight line, where the slope, y-intercept and the lineal correlation coefficient are shown in figure 1, whose values were 0.270, 58.66, and 0.147 respectively. A low correlation was obtained between both data sets (r = 0.38) and the calculation of the concordance correlation coefficient which similarly showed a low concordance between Phe levels for IVS and VS (rc = 0.36). Values r12 and c12 obtained were 0.40 and 0.95, which evidences that the poor quality of samples affects more value precision rather than its accuracy. Mann Whitney´s U Test application showed that no meaningful statistical differences existed when IVS compared to VS (p = 0.12).
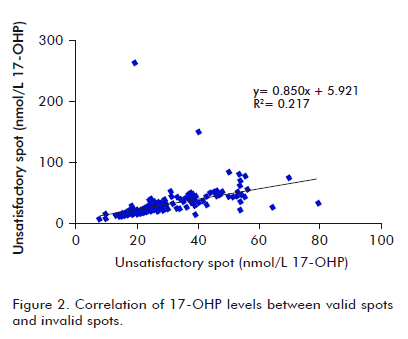
A similar evaluation was made for 17-OHP levels. Mean concentration values were 28.6 and 30.5 nmol/L for VS and IVS, respectively. The regression curve was y = 0.850x + 5.921, for a lineal correlation coefficient of 0.217, showing a low correlation between both sets of data (r = 0.47), as shown in figure 2. There was a low correlation concordance for 17-OHP levels to VS and IVS (rc = 0.39). r12 and c12 were 0.46 and 0.84, respectively, supporting the higher incidence of low sample quality on precision of values, and accuracy to a lower extent.
P value obtained at the mean comparison study between IVS and VS was 0.84, which corroborates that no meaningful statistical differences existed for a 95% trustability interval.
Newborn samples presenting stains somewhat invalid (n = 592) were simultaneously classified as to their appearance in: IS (27%), CS (26%), RS (23%), CBS (16%) and SSS (8%).
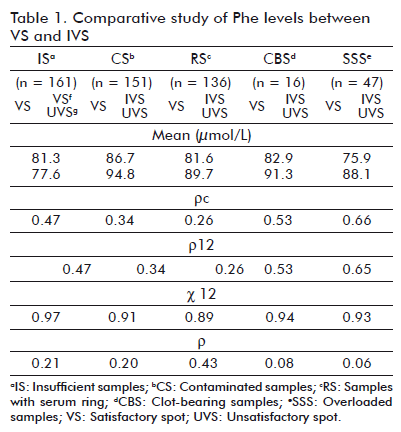
Phe mean concentration values and 17-OHP for VS and IVS were calculated separately in each group. Similarly was conducted for the calculation of stadigraphs r12 and c12 and for Pearson correlation coefficient and correlation concordance. Tables 1 and 2 show the corresponding values for each of the studied analytes.
Is exhibited a decrease in mean concentration values for both analytes in IVS compared to VS from the same sample; meanwhile CBS, SSS and RS showed higher mean levels and control stains. In CS, Phe mean values increased 9% and those of 17-OHP were similar to control stains.
Is presence relates with the extraction and an incorrect blood drop formation when collected in filter paper. To avoid such phenomena, an increase in venous pressure and working with lancets with a depth less than, or equal to 2 mm should be favored (4), the application of blood with a capillary tube or any other device, or removing the filter paper before blood thoroughly fills up the circle, or before it could be absorbed on the other side of the paper should be avoided. Finally, before or after sample obtainment, touching filter paper straight with the hands or leaving it in close contact with substances like hand lotion, powder or any other should be impeded.
Phe levels and 17-OHP in SSS and CBS augment as a consequence of filter paper contact with blood drops more than once, which is conditioned by the application of an excess of blood (probably with a device) or by adding blood to both sides of the filter paper. Likewise, in RS an increase in both analytes´ levels was observed. A splitting of serum and erythrocytes was spotted which could relate to the presence of an excess of the disinfectant agent and precipitate blood components affecting the homogeneous migration on filter paper, On these cases, the area should entirely be dried before cutaneous punction, not excessively compressing the zone surrounding the punction area and avoid the use of capillary tubes for the application of blood and the contact of filter paper with contaminant substances. This invalidation cause has also been associated to incorrect sample drying procedures. Besides, in the case of CS, sample exposure to heat should be avoided.
Discordant results were detected among some IVS with false positive and negative values in respect to control stains. In Cuba, a 240 mmol Phe/L whole blood limit value is accepted for the diagnostics of phenylketonuria at early ages. Samples presenting an equal or superior concentration are considered as elevated. Similarly, for the diagnostic of congenital adrenal hyperplasia a 55 nmol per L of whole blood value is recognized. In four samples, Phe concentration values from IVS were higher than 240 mmol Phe/L, while Phe concentration value from VS were less than the cut-off level. A similar result was observed for 17- OHP levels in two samples.
From the methodological point of view, false positive results lead to sample re-testing and in most of cases, sample re-collection. A high rate of false positive values not only increases the real cost of the NS, but also promotes psychological stress among parents of the affected children (15). Therefore, NS programs need to control all possible sources of incorrect results.
Additionally, in two samples, Phe levels from IVS were less than the cut-off level, while the values in control spots were out of range. For 17-OHP levels, three samples with similar parameters were found.
Obtaining false negative results in laboratories avoid an early diagnostic and if treatment at the neonatal period is not applied, clinical signals of the disease appear.
Filter paper samples are a valuable source of information; nevertheless their reliability depends on the stability of analytes in the stored samples (16, 17).
Our results confirm that assays using filter paper dried blood samples are susceptible to be affected due to several factors (16, 17). The sample quality is one of these factors, therefore, is essential to evaluate and guarantee adequate procedures starting at sample collection until laboratory analyses. Other studies have also shown the correct application of sample collection procedures which have proved being fundamental to obtain reliable results in NS laboratories (18-20).
Sample collection must be properly conducted to avoid an insufficient or "non-useful" sample. Quality control rules require rejecting the samples unfulfilled with the established requirements. The sample can be rejected during the receipt process due to unsatisfactory properties in the spots, or while carrying out the test due to partial sample elution or no elution at all; the eluate shows colorless or pale color depending to the denaturalization of hemoglobin and even the blood disc shows dark color.
An unsatisfactory sample will be rejected by the laboratory, needing a second collection of the sample. This procedure generates discomfort to the newborn, distress to the parents and delay of the diagnostics. That is the reason why increasing precautions is necessary during sample drying, storage and transportation.
U.S´s NCCLS described a methodology for collecting, drying and preserving blood samples on filter paper (6). Among the main procedures for adequate collection are: writing the identification of the patient without omitting information, avoiding touching the clean circle in the filter paper before the sampling; cleaning the heel with 70% alcohol and removing the excess of solvent with cotton or others; using of sterile material during the procedure; removing the first drop of blood and applying the next one on the filter paper; assuring the blood be applied on a single side of the paper and reach the other side (4, 6).
Once the blood drops are collected, the filter paper cards must be placed in a device allowing the drying, horizontally suspended to avoid contact with other surfaces and promoting a homogeneous diffusion of the blood drop. Filter paper cards must be dried at 20- 25 °C during three hours without exposure to sunlight and avoiding the contact with antiseptic solutions or any other materials and stored in clean places. Cards would never be dried in microwave ovens, stoves or by any other source of artificial energy.
The correct drying procedure is important due to moisture may damage sample quality and induce bacterial growth o alter the sample elution time.
Once dried, the cards must be placed at a 180º relative position from each other, to avoid cross-contamination among the blood spots from different samples. They must be stored properly identified in paper envelopes. It is advisable that samples are protected with desiccant in plastic bags during their transportation and storage in freezers (2 - 8 ºC), to avoid they could humidify in case of freezing malfunction or by accident during transportation.
Filter paper of the ones established by the NS program must be used (17), which own special physicalchemical properties, and support adequate absorption, retention and homogeneity. These parameters are essential to obtain reliable results. Unused cards must be stored at a clean place and protected free from dust to avoid contamination.
Finally, once dried, samples must be sent as fast as possible to the laboratories under the recommended packing conditions. Packages must avoid heat or water exposure and guarantee their integrity (17).
The quality of the dried blood samples on filter paper is a key step for NS; therefore, the normalization of procedure and the integral training of the personal are of extreme importance to guarantee the effectiveness of the programs (16). Knowing how the properties of the sample, their preservation, transportation and distribution affect the analytical assay is a must to obtain reliable results in NS laboratories (16).
Knowing the properties of the sample, its preservation, transportation and distribution, determines the outcome of the analytical assay, because are key elements to obtain reliable laboratory results.
REFERENCES
1. Raimann BE, Cornejo EV. Una primera aproximación al diagnóstico y tratamiento de errores innatos del metabolismo. En: Colombo CM, Cornejo EV, Raimann BE, eds. Errores innatos en el metabolismo del niño. 1a ed. Santiago de Chile: Editorial Universitaria; 1999, p.45-56.
2. Wilcken B, Wiley V. Newborn screening. Pathology 2008; 40(2): 104-15.
3. Waite KV, Maberly GF, Eastman CJ. Storage conditions and stability of thyrotropin and thyroid hormones on filter paper. Clin Chem 1967;33(6):853-5.
4. Mei JV, Alexander JR, Adam BW, Hannon WH. Use of filter paper for the collection and analysis of human whole blood specimens. J Nutr 2001; 131: 1631S-6S.
5. Torök D, Mühl A, Votava F, Heinze G, Sólyom J, Crone J, et al. Stability of 17a-hydroxyprogesterone in dried blood spots after autoclaving and prolonged storage. Clin Chem 2002;48(2):370-2.
6. Hannon WH, Boyle J, Davin B, Marsden A, McCabe ERB, Schwartz M et al. Blood collection on filter paper for neonatal screening programs, 3rd ed, approved standard, NCCLS Document A4-A3. Wayne, PA: NCCLS, 1997.
7. Kaye CL and the Committee on Genetics. Newborn screening fact sheets. Pediatrics 2006;118:934-63.
8. Heredero L, Atencio G, Vega JL, Gutiérrez E, Damiani A. Early diagnosis of phenylketonuria in Cuba: preliminary report. Rev Cubana Pediatr 1986;58:27-33.
9. Martínez L, Robaina Z, García S, Gutiérrez E. The Cuban program for neonatal screening of phenylketonuria. Twenty years of experience: the clinical and social attention. Rev Cubana Genet Comunit 2008;2:45-51.
10. Antal Z, Zhou P. Congenital adrenal hyperplasia: Diagnosis, evaluation, and management. Pediatr Rev 2009;30: e49-e57.
11. White PC, Speiser PW. Congenital adrenal hyperplasia due to 21-Hydroxylase deficiency. Endocr Rev 2000; 21(3): 245-91.
12. González EC, Frómeta A, del Río L, Castells E, Robaina MS, García SM, et al. Cuban neonatal screening of phenylketonuria using an ultramicro-fluorometric test. Clin Chim Acta 2009;396:129-32.
13. González EC, Marrero N, Pérez PL, Frómeta A, Zulueta O, Herrera D et al. An enzyme immunoassay for determining 17a-hydroxyprogesterone in dried blood spots on filter paper using an ultramicroanalytical system. Clin Chim Acta 2008; 396:63-6.
14. Lin LI. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989;45:255-68.
15. Olgemöller B, Roscher A, Liebl B, Fingerhut R. Screening for congenital adrenal hyperplasia: adjustment of 17- hydroxyprogesterone cut-off values to both age and birth weight markedly improves the predictive value. J Clin Endocrinol Metab 2003;88(12):5790-4.
16. Cedillo B, Estrada RA, Jonguitud V, Parra I. Factores que afectan algunas de las pruebas del tamiz neonatal. Med Univer 2007;9(34):3-6.
17. Frómeta A, Marrero N, González E, Lugo E, Fernández L, Doménech M. Estudio comparativo de los tres papeles de filtro en los ensayos UMELISA para el tamizaje neonatal de hipotiroidismo congénito y fenilcetonuria. Rev Biomed 2002; 13:241-7.
18. Dezateux C. Evaluating newborn screening programmers based on dried blood spots: future challenges. Br Med Bull 1998;54:877-90.
19. Pappaioanou M, Kashamuka M, Behets F, Mbala S, Biyela K, Davachi F, et al. Accurate detection of maternal antibodies to HIV in newborn whole blood dried on filter paper. AIDS 1993;7(4):483-8.
20. Meites S, Glassco KM. Studies on the quality of specimens obtained by skinpuncture of children. 2. An analysis of blood collecting practices in a pediatric hospital. Clin Chem 1985;31(10):1669-72.
Received in April, 2010.
Accepted for publication in December, 2010.