My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.27 no.4 La Habana Oct.-Dec. 2010
REPORT
Chitosans as bioactive macromolecules to protect conomically relevant crops from their main pathogens
Alejandro Falcón Rodríguez1, Aida T Rodríguez2, Miguel A Ramírez2, Deyanira Rivero2, Benedicto Martínez3, Juan C Cabrera4, Daimy Costales1, Ariel Cruz2, Luis G González5, María C Jiménez5, Leonel Jiménez5, Ileana Hernández1, Dianevys Gonzáles Peña1, Ramona Márquez1
1 Departamento de Fisiología y Bioquímica Vegetal, Instituto Nacional de Ciencias Agrícolas La Habana, Cuba
2 Estación experimental del Arroz, Instituto Nacional de Ciencias Agrícolas Los Palacios, Pinar del Río, Cuba
3 Departamento de Protección de Plantas, Centro Nacional de Sanidad Agropecuaria, La Habana, Cuba
4 Departamento de Biología Celular Vegetal, Facultad de Agricultura, Universidad Notre Damme de la Paix Namur, Bélgica
5 Facultad de Agronomía, Universidad de Granma Cuba
ABSTRACT
Studies were carried out as part of the Agriculture Biotechnology program, to prepare and characterize chemically and biologically different chitosans obtained from Cuban lobster chitin. Chitosan polymers were subjected to acid and enzymatic hydrolysis by using low·cost commercial enzymatic preparations, and the resulting oligosaccharide mixtures were further characterized. Their potential antimicrobial activities were also evaluated versus fungi and oomycetes, also testing their ability to induce defensive and protective responses in tobacco and rice plants against two economically relevant pests, Phytophthora nicotianae and Pyricularia grisea, respectively. With the aid of international collaboration, different oligochitosans mixtures were compared for activating defensive responses in suspension cultures of Arabidopsis thaliana cells. These results bring knowledge on the physical·chemical properties of the chitosans obtained, such as molar mass and acetylation grade, and their influence on activating defensive responses, the inhibition of growth in pathogens and the induction of resistance in tobacco and rice plants. Some of these chitosan derivatives were selected as possible active components to protect both type of cultivars, being applied at field·scale to evaluate their effects for the main natural pathogens and bringing very promising results. This research allowed us to establish a methodology for preparing oligochitosans, and results shown inhere were part of BSc, MSc and PhD theses, and were also published in more than 20 scientific papers and presented in more than 40 scientific conferences.
Keywords: chitosan, enzymatic hydrolysis, antifungal activity, induced resistance, Phytophtora nicotianae.
INTRODUCTION
Higher plants are able to initiate defensive reactions in response to pathogen´s infections [1]. Those responses are triggered when the plant recognizes several signals which are released as products of the mutual enzymatic degradation of cell walls of both, the plants and the pathogens [2]. The resulting oligosaccharides, also known as oligosaccharins, not only serve as primary signals of the defensive responses, but also influence other biological responses regarding vegetal growth and development [3, 4]. Among oligosaccharins, the cell wall pectins´ oligogalacturonides, b-glucans, and the fungi cell wall chitin and chitosan fragments are potential inducers of resistance and protection from diverse pathogens in plants. That´s the reason why they are currently included as active components in several protective products for agriculture [3-6].
Chitin is a linear polymer of N-acetyl-glucosamine, and the second more abundant natural polymer after cellulose. It is found on fungi cell walls and exoskeletons of arthropods as the main production source worldwide, with more than 10 gigatons (1 x 1013 kg) produced per year. It is mainly obtained from shrimp and crab exoskeletons, with a very small production from lobster exoskeletons [7]. The latter are discarded in large amounts as a byproduct to the sea to avoid environmental problems, which could be used to obtain macromolecules highly applicable in medicine, industry and agriculture [7].
Chitosan, the main chitin derivative, is a linear polymer of glucosamine monomers linked by b 1-4 bonds and it is obtained by alkaline deacetylation. This process produces a polymer soluble in diluted acids, which is advantageous over chitin for agricultural use. On this field, chitosans and their derivatives of lower molecular weights could be applied, based on their proven biological potentialities, such as signifi-cant antimicrobial activity on the growth and development of fungi, bacteria and oomycetes [5, 8]; protec-tion of plants from potential pathogens and promotion of cultivar growth and development [5, 6].
Both, the direct antimicrobial activity and the induction of defensive responses against pathogens of chitosans, are influenced by the physical-chemical properties of the molecule, including the acetylation degree (AD) and molecular weight (MW) [5, 9]. The lower AD of the given chitosan polymer the higher inhibitory activity on microbial growth is obtained [5]. On the contrary, a decrease a decrease in the MW of the given chitosan may increase or decrease its inhibitory activity on growth, depending on the specie [5, 10]. These types of studies are scarce on oomycetes, and there are no studies in literature analyzing the influence of these two variables on the development of this genus.
These properties also influence the activation of defensive responses in plants. Kauss and coworkers [11] demonstrated that partial acetylation and fragmentation of chitosans were required for inducing H2O2 in cucumber. On the other hand, results from Vander et al. [9] indicated that chitosan polymers with AD over 35% induced the highest Phenylalanine ammonia lyase (PAL) and peroxidase (POD) levels in wheat leaves. Previous works on the role of AD and MW for chitosan-based induction of defensive responses were carried out only in isolated parts of pre- [9] or non-conditioned [11] plants but neither in complete plants nor in the presence of pathogens.
Even when there are reports of the protection of cultures against pathogens by administering different types of chitosans [5, 12], the effect of this compound on the Nicotiana tabacum-Phytophthora nicotianae interaction remains to be elucidated, being only reported the partial protection against the Tobacco Mo-saic Virus (TMV) by using oligochitosans mixtures [13]. Therefore, there was no information about the induction of resistance against pathogens other than the TMV in tobacco plants, nor in the evaluation of chitosan for inducing responses in Cuban tobacco and rice species.
Given the current context of Cuban agriculture, it is highly relevant to have a non-toxic natural product in origin, produced from national sources and by affordable means, as a replacement alternative for the expensive chemical pesticides being imported.
The Group of Bioactive Products (GPB) at the National Institute of Agricultural Sciences (INCA) has experience in developing methodologies to prepare oligosaccharins from national raw materials and eva-luating them as hormone substitutes for in vitro culture, to promote root anchoring, soybean symbiosis improvement and induction of plant resistance against diseases. Several of these results have been already patented.
Based on what mentioned above, our research of chitosans focused on the following objectives: develo-pment and adaptation of methodologies to obtain chitosans polymers and oligomers from nationally-pro-duced lobster chitin, which show potential biological activity in plants; to evaluate the effect of chitosans of different AD and MW in the in vitro development of pathogens of economically relevant crops; to evaluate the effect of chitosans in inducing resistance in suspension cultures of Arabidopsis cells, and in tobacco and rice plants inoculated with pathogens and; to test promising compounds on field experiments for both crops.
MATERIALS AND METHODS
Preparation of chitosan poly- and oligosaccharides
Chitosan polymers were obtained by basic deaetylation with NaOH at reactor scale, from nationally obtained lobster chitin. The methodology formerly described to obtain shrimp chitin was adapted for lobster chitin processing at the GBP (Group of bioactive products) of INCA. Studies were carried out by applying chitosan hydrolysis with commercial enzymatic mixes and using typical enzymology techniques, such as: the influence of different substrates at different concentrations, pH, temperature and others. Chitosan mixtures were characterized using mass spectrometry technique (MALDI-TOF) for the first time to determine oligosaccharide AD and MW simultaneously. Acid hydrolysis [14] was also tested to obtain oligochitosans.
Analysis of the effect of chitosans on pathogen growth
The effect of chitosan-based compounds on mycelial growth, sporulation and spores viability were analy-zed. Pathogens were isolated from rice and tobacco plants, and cultured in petri dishes filled with solid culture media (PDA, PDA-V8 and/or Czapek Dox culture media) containing the tested chitosan com-pounds. Spores were incubated with chitosans prior to culturing them on chitosan-free media or by direct germination of spores in chitosan-containing aqueous solutions.
Evaluation of chitosan-induced plant resistance
The activation of defensive responses was studied in suspension cultures of Arabidopsis thaliana cells, and in leaves and roots of tobacco and rice plants. PAL induction and the increase of H2O2 production in response to chitosan oligosaccharides were determined. According to each experiment, b 1-3 glycanase, chitinase, PAL and POD enzymatic activities were assessed in leaves and roots of tobacco and rice plants. Additionally, the induction of resistance by applying chitosans of different AD and MW was determined in tobacco seedlings against Phytophthora nicotianae (Pn) at bioassay scale. It was also tested in rice, by immersing the seeds into a solution containing chitosan polymers and oligomers prior to sowing, and fur-ther inoculating the leaves of the resulting seedlings with Pyricularia grisea.
Field-scale application of chitosans
Field experiments were carried out in tobacco (variety Habana 92) and rice (variety J-104) cultivars under productive conditions. Tobacco plants were applied with compounds, by foliar aspersion of a chitosan polymer following plant transplantation, and rice cultivars were established by immersing the seeds into a solution containing a chitosan polymer and a chitosan hydrolysate prior to sowing. The natural infection by major pathogens for these two crops was evaluated as indicated on the instructives for infection scales and analyses.
RESULTS AND DISCUSSION
A methodology similar to that described for obtaining chitosan from shrimp chitin was used to obtain chitosan polymers at bank scale. The technique was modified according to a previous study of the influence of chitin/alkali ratio, temperature and reaction time on the properties of the chitosan obtained. Results showed that it is possible to generate high molar mass chitosans under homogeneous preparation conditions (low temperature and an almost equivalent alkali/chitin ratio), with minor modification of its secondary structure and with yields between 74 and 80% of the source chitin. All these are economically relevant for an efficient use in agriculture.
On the other hand, both acidic and enzymatic hydrolyses were studied to generate low MW chitosan compounds. Currently, the enzymatic hydrolysis is preferred over the acidic one, due to its sequence specificity for lysis and the respective formation of products [7]. Nevertheless, the costs of the enzymes used must be low enough to make it affordable for agricultural application and to obtain products with the proper biological activity. The first studies using this strategy showed that it is possible to generate chi-tosan hydrolyzates having protective activity in plants, by using low cost, commercial enzyme mixes, to improve the biological activity of the obtained chitosan derivatives. In our study, five enzymatic complexes were assayed; allowing us to select the most adequate and it was possible to optimize the hydrolysis conditions (Figure 1). Hydrolyzates and their mixes were obtained with high content of bioactive oligosaccharides and containing the selected complex (Pectinex Ultra SPL)[14].
The biological activity of chitosan oligosaccharides depends on its structure. Otherwise, previous reports did not addressed the influence of AD on the activity of quito-oligosaccharides while in others the AD was not determined or ambiguous methods were used to characterize it.
In this work, chitosan was depolymerized by aciacidic or enzymatic hydrolysis, by using the Pectinex Ultra SPL complex. The resulting oligosaccharides were selectively precipitated in methanol solutions and rigorously characterized by mass spectrometry (MALDI-TOF). The differences in the polymerization degree (PD) and AD were well established (Table 1). The acidic hydrolysis produced fragments with PD up to 16 glucosamine residues, mostly monoacetylated. More significantly, the enzymatic hydrolysis rendered shorter fragments with a high rate of fully deacetylated chitooligomers, showing a higher oligo size/yield ratio. Based on these evidences, we chose the enzymatic method to prepare oligochitosans [14].
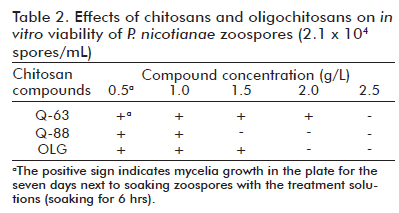
Among the biological assays, the effect of different chitosans on growth and asexual reproduction of tobacco and rice pathogens was evaluated in vitro. Significantly novel results were obtained regarding the effect of chitosans at different lifecycle stages of the Sarocladium and Bipolaris genera. Similarly, it was evidenced the influence of chitosan MW and AD on the different stages of the Phytophthora nicotianae tobacco pathogen, achieving the highest inhibition by decreasing MW down to oligomers and at the lowest AD. (Figure 2, Table 2). This is a highly significant result, due to its practical relevance when preparing chitosan compounds with inhibitory activity.
It was also evaluated the influence of a chitosan polymer (Q-88) on other pathogens growth (Phytium aphanidermatum, Sclerotium rolfsii and Rizoctonia solani) which were isolated from tobacco and other solanaceae, with the first two pathogens as the most sensitive to that polymer, and studying Q-88 for the very first time in dose-response experiments.
Based on these results, and also considering the natural origin and biodegradable nature of chitosan, these polymers and their derivatives were recommended to be used for controlling soil phytopathogens in tobacco seedbeds and to reduce the spotted grain disease in rice.
It was also demonstrated that it is possible to re-acetylate the high PD polymers without affecting their PD, by working with two sets of chitooligosaccharides of defined PD. Their capacity to induce resistance was evaluated in suspensions of Arabidopsis thaliana cells, by measuring the induction of two well known defensive markers: PAL activity and production of hydrogen peroxide (H2O2). In this case, the completely deacetylated chitooligosacharides induced the activation of both PAL activity and H2O2 production, and also cell death, which varied with their PD and concentration. The ability of the oligosaccharides to increase H2O2 production and cell death was progressively inhibited by reacetylation, but the PAL activity was unaffected. This evidenced the role of PD for generating defensive responses on vegetal cells.
Experiments run in tobacco plants showed, for the very first time, the induction of systemic responses against P. nicotianae by foliar aspersion, substrate application and previous treatment of seeds with the chitosan compounds. All these demonstrate the potentiality of these compounds to protect tobacco from its main disease at seedbed level. The induction of resistance, either by activating defensive responses or by reducing the infection of P. nicotianae was influenced by the concentration, MW and AD of the applied compounds, and also by the administration procedures [15].
This study evidenced the benefits of protecting tobacco at field scale under semi-controlled conditions, and also the practical relevance of preparing chitosans with the adequate physical-chemical properties, to enhance the protective effect.
In rice, the treatment of seeds of the commercial variety J-104 sensitive to Piriculariosis (Pyricularia grisea) with chitosan polymers or hydrolizates prior to sowing, activated defensive responses in plants either or not inoculated with the pathogen, protecting the plants against infection. These assays were carried out under semi-controlled conditions and demonstrated that it is possible to protect this crop from this significant disease at field scale with both types of compounds.
The most promising among all the chitosan-based compounds studied in this work at field scale and under controlled or semi-controlled conditions were assayed at open field scale in both crops. In rice, both the polymer and the hydrolizate were studied, under conditions favoring the occurrence of Piriculariosis, with protection being evidenced only for the chitosan hydrolizate at the concentration assayed.
In tobacco, taking together the results of resistance induction and antimicrobial activity, it was decided to evaluate a chitosan polymer (Q·88) by foliar aspersion to plants (variety Habana 92), several days after transplantation and without altering the protection schedule used by the local private producer. In this sense, plants received the chitosan (three doses) and also the scheduled chemical treatment, being finally evaluated for natural infection by viruses, fungi and oomycetes. A significant protection was obtained against all the evaluated pathogens as compared to the control, showing a dose-dependent response for the applied chitosan and also a positive effect of the compound on production yields.
Due to its dual biological action, chitosan-mediated protection of plants can be mediated both by directly affecting pathogen growth and by activating the systemically induced resistance (SIR) in plants. In rice, where the seeds were treated before sowing, the protection found was the second in magnitude. But in tobacco, where the compound was disseminated by aspersion, both effects can be taking place, the antimicrobial action (especially against air-borne pathogens which can get into contact the chitosan adhered to the leave) and the SRI. The latter evidenced by the reduced infection with root and stem pathogens after aspersion. Both mechanisms of action are vital to implement a protective strategy in crops.
CONCLUSIONS
In this work, methodologies were adapted to generate different chitosan compounds from lobster chitin. The resulting and promising polymers, and their mixes and derivatives, were evaluated for biological activity and activation of resistance and protection from pathogens in tobacco and rice plants. It was demonstrated, for the very first time, the influence of certain physical-chemical properties of chitosan derivatives on inhibiting pathogens growth and development and also in the biology of protection of fully grown plants against them. Our results evidenced the structure-activity relationship among the derivatives prepared and characterized and also the biological functions evaluated.
The first results on protection of rice and tobacco cultivars at field scale are also shown, evidencing their relevance to design and prepare new natural, non-toxic and nationally produced bio-pesticides to be used in agriculture. The inclusion of chitosan as part of the integral management of pathogens would allow reducing or substituting the application of chemicals in some economically relevant crops, with the subsequent protection of the environment.
SCIENTIFIC RELEVANCE OF THE STUDY
This work provides evidences on the potentialities of chitosan polymers, partially hydrolyzed chitosan and oligochitosans developed in Cuba, to protect tobacco and rice cultivars from relevant diseases which limit production yields. This supports their potential introduction for the ecological control of pathogens in both crops.
By these means, a significant amount of lobster exoskeleton is used as source for chitin and chitosan production, two compounds of high aggregated value, instead of discarding it into the environment with the associated contamination.
ACKNOWLEDGEMENTS
The authors want to thank the contribution and support of the following collaborators: The technical team at the INCA involved in this work; Dr. Pierre Van Cutsem (Namur University, Belgium), Dr. Silvia Bautista-Baños (IPNY, Morelos, Mexico); Dr. Miguel A Martínez-Téllez (CIAD, Sonora, Mexico); Dr. Fernando Guridi (UNAH); Dr. Eduardo Ortega (University of Havana, Cuba); Dr. Ondina León (CENSA, Cuba); Dr. Verónica Toledo (IIT, Cuba) and Dr. María C. Nápoles (INCA, Cuba). The authors are also grateful to MSc. Regla M Cárdenas and MSc. Elizabeth Cristo; and to the CITMA and MES ministries for partially funding the research included in this work.
REFERENCES
1. Agrios GN How plants defend themselves against pathogens (Chapter six). In: Plant Pathology. 5th ed., New York, USA: Academic Press, 2005, p. 208.
2. Esquerré-Tugayé MT, Boudart G, Dumas B. Cell wall degrading enzymes, inhibitory proteins, and oligosaccharides participate in the molecular dialogue between plants and pathogen. Plant Physiol Biochem 2000;38:157-63.
3. Ridley BL, O´Neill MA, Mohnen D. Pectins: structure, biosynthesis, and oligogalacturonides-related signaling. Phytochemistry 2001;57:929-67.
4. Shibuya N, Minami E. Oligosaccharide signalling for defenses responses in plant. Physiol Mol Plant Pathol 2001;59:223-33.
5. Bautista-Baños S, Hernández-Lauzardo AN, Velázquez-del Valle MG, Hernández- López M, Ait Barka E, Bosquez-Molina E, et al. Chitosan as a potential natural compound to control pre and postharvest diseases of horticultural commodities. Crop Prot 2006;25:108-18.
6. Sharathchandra RG, Niranjan Raj S, Shetty NP, Amruthesh KN, Shetty HS. A Chitosan formulation ElexaTM induces downy mildew disease resistance and growth promotion in pearl millet. Crop Prot 2004;23:881-8.
7. Prashanth KVH, Tharanathan RN. Chitin/chitosan: modifications and their unlimited application potential-an overview. Trends Food Sci Technol 2007;18:117-31
8. Xu J, Zhao X, Han X, Du Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pest Biochem Physiol 2007;87:220-8.
9. Vander P, Varum KM, Domard A, El Gueddari NE, Moerschbacher BM. Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves. Plant Physiol 1998;118:1353-9.
10. Jeon Y-J, Shahidi F, Kim SK. Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev Int 2000;16:159-76.
11. Kauss H, Jeblick W, Domard A, Siegrist J. Partial acetylation of chitosan and a conditioning period are essential for elicitation of H2O2 in surface- abraded tissues from various plants. In: Domard A, Roberts GAF, Vårum KM (eds), Advances in Chitin Science II, Lyon, France,: J André Publisher, 1997, p. 94–101.
12. Trotel-Aziz P, Couderchet M, Vernet G, Aziz A. Chitosan stimulates defense reactions in grapevine leaves and inhibits development of Botrytis cinerea. Eur J Plant Pathol 2006;114:405-13.
13. Zhao XM, She XP, Du YG, Liang XM. Induction of antiviral resistance and stimulary effect by oligochitosan in tobacco. Pest Biochem Physiol 2007;87:78-84.
14. Cabrera JC, Van Cutsem P. Preparation of chitooligo·saccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem Eng J 2005;25:165·72.
15. Falcón AB. Chitosanas en la inhibición in vitro de Phytophthora nicotianae Breda de Haan y en la inducción de resistencia en plantas de tabaco contra este patógeno. Doctoral Thesis in Biological Sciences. Faculty of Biology, University of Havana, July 2009.
Alejandro Falcón Rodríguez, Departamento de Fisiología y Bioquímica Vegetal, Instituto Nacional de Ciencias Agrícolas La Habana, Cuba. E-mail: alfalcon@inca.edu.cu