My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.28 no.2 La Habana Apr.-June 2011
REPORT
Protective immune response against Neisseria meningitidis serogroup B after immunization with peptides mimicking a capsular polysaccharide epitope
Tamara Menéndez, Hilda E Garay, Osvaldo Reyes, Yoelys Cruz, Edelgis Coizeau, Nelson F Santiago, Glay Chinea, Karem Cobas, Evelyn Caballero, Vivian Morera, Jesús Noda, Tania Carmenate, Yordanka Soria, Emma Brown, Alejandro Martín, Daniel Yero, Sonia González, Gerardo E Guillén
Centro de Ingeniería Genética y Biotecnología, CIGB Ave. 31 e/ 158 y 190, Cubanacan, Playa, AP 6162, La Habana, Cuba.
INTRODUCTION
The Gram-negative bacterium Neisseria meningitidis is an important cause of meningitis and septicemia worldwide. Meningococcal disease is a life threatening illness that may progress to death even after medical intervention. Approximately 500 000 - 1 000 000 cases of invasive meningococcal disease occur annually worldwide with a 10% mortality rate. Significant sequelae, including neurological damage, limb loss and hearing loss, occur in up to 20% of survivors. Meningococcal disease is more common among infants and young children (1). Prevention through vaccination remains the most effective approach to control invasive meningococcal disease.
The most relevant mechanism for antibody-mediated protection against N. meningitidis seems to be the antibody-mediated direct complement lysis (2). The in vitro measurement of complement-dependent serum bactericidal activity (SBA) is currently considered the immunological surrogate of protection against meningococcal disease (3).
N. meningitidis is classified in 13 serogroups, based on chemical composition and antigenic differences of the capsular polysaccharide (CPS). Serogroups A, B, C, Y and W-135 strains account for most cases of meningococcal infections (1). Effective plain or conjugated CPS-based vaccines are available against serogroups A, C, Y and W-135 (1). However, a vaccine inducing protection against most of the circulating variants of serogroup B meningococcal strains is not yet available.
Up to now, all field-applied anti-B vaccines have been based on outer membrane vesicles (OMV), which vary in composition among strains. Therefore, OMV-based vaccines induce an immune response mainly protective against the strain used for vaccine production and control the disease in specific regions with similar circulating strains. The vaccine VA-MENGOC-BC®, developed at the Finlay Institute (Havana, Cuba), is an OMV-based vaccine that uses the Cuban type strain CU385 (B:4:P1.19,15; ST=33). The vaccine showed an efficacy of 83 % in clinical trials and its introduction into the Cuban Immunization Program keeps low the incidence of meningoccoccal disease in Cuba (4).
The poor immunogenicity of purified MenB CPS, even after conjugation (5) has precluded the formulation of vaccines based on this compound. MenB CPS is a linear homopolymer of a(2-8)-linked N-acetyl neuraminic acid (polysialic acid, PSA) (6). The mammalian neural cell adhesion molecule (NCAM) is glycosylated with identical sialic acid residues (7). The replacement of N-propionyl (Npr) groups for N-acetyl (NAc) moieties in MenB CPS greatly increases immunogenicity and allowed the discovery of two classes of antigenic determinants in the MenB CPS, which can be found on the intact bacterial surface: human non-cross-reactive and human cross-reactive determinants (8-10).
The identification of peptide mimetics of MenB CPS-specific antigenic determinants is a strategy aimed to further the development of vaccine formulations to cover all meningococcal serogroup B strains. In the present work we investigated the possibility to identify peptides able to mimic the properties of a unique capsular polysaccharide epitope in MenB that could be proposed as meningococcal serogroup B vaccine candidates.
RESULTS
To identify mimetic peptides of meningococcal serogroup B capsular polysaccharide, a collection of random peptides was screened, exposed in the surface of filamentous phages (11) using the bactericidal and protective IgG2a mAb 13D9 (10) as bait. Four peptides, able to bind mAb 13D9 in competition with MenB CPS, were identified and named 4L-5, 2L-4, 2L-17 and 2L-27.
The alignment and comparison of the four sequences (Figure 1) revealed the presence of a 7-residue stretch in each of them displaying some of the common residues found in other carbohydrate-mimicking peptides, with coinciding relative positions (12, 13). One of these features is the presence of W (tryptophan) residues. Here a W residue was found in 3 out of the 4 peptides in a very similar sequence context. W, and aromatic amino-acids in general, are hypothesized to mimic monosaccharide moieties due to similarities in cyclic shape and volume. Another signature of carbohydrate-mimicking peptides is the presence of P (proline) residues, which are thought to function by introducing turns in the peptide backbone that allow the adoption of an adequate spatial disposition by the voluminous side chains of aromatic residues, resembling the carbohydrate they mimic. There is more than one P residue in all of the sequences identified here, and in three cases a P residue appears in a very similar sequence context. E (glutamic acid) is also present in the four peptides. An E residue in the same sequence context can be found for peptides 4L-5, 2L-4 and 2L-17, but is apparently missing in peptide 2L-27. However, even in this case the C-terminal E might be considered to be present in a similar sequence context, since it is preceded by a turn-inducing sequence (TNE) that should bring the E into close spatial proximity to the consensus stretch. Generally, the presence of acid residues in carbohydrate-mimicking peptides guarantees charge similarity with negatively charged polysaccharides, as is the present case with PSA.
It was hypothesized that this 7-residue region in each peptide could be the target for mAb binding, and that probably the E plays a role in such binding. The 4L-5 sequence was selected as a model to test this hypothesis. Using collections of overlapping peptides covering the 4L-5 sequence it was defined that the sequence WYVE, contained in the afore mentioned 7-amino-acid-residue stretch, sufficed for mAb binding. A synthetic peptide containing three copies in tandem of the minimal sequence necessary for mAb 13D9 binding in peptide 4L-5 inhibited mAb 13D9 binding to MenB CPS. The importance of the E residue for mAb binding was studied with synthetic peptide libraries containing amino acid changes in the 4L-5 sequence. It was found that the E residue is essential for mAb binding since its replacement by other amino acids abolished the reactivity with the mAb. These experiments also revealed the role of the two prolines located at positions -3 and -2 counting from W in WYVE, because their presence increased the reactivity of peptide 4L-5 with mAb 13D9, even though they are not directly involved in binding.
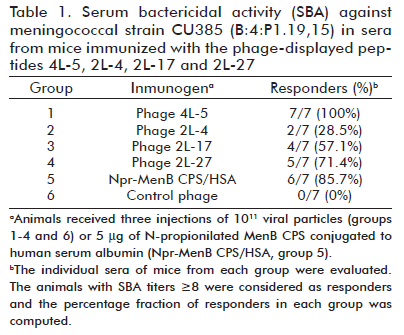
To assess the potential of the identified peptides as vaccine candidates, we started immunizing groups of mice directly with the viral particles displaying such sequences. For three of the phage-displayed peptides (4L-5, 2L-17 and 2L-27), more than half of the mice from the corresponding group elicited bactericidal specific anti-peptide IgG antibodies (Table 1). The highest bactericidal titers were measured in the group immunized with phage-displayed 4L-5 and passive transfer of pooled sera from this group conferred protection to infant rats challenged with live MenB.
The 4L-5 sequence was selected as a model to develop vaccine formulations, based on synthetic peptides, that could be used in humans. Various forms of sequence presentation to the immune system were evaluated (Figure 2). The structures L/4L-5; MAP 4L-5 and D/MAP 4L-5 were conjugated to the carrier protein P64K. Three conjugation methodologies were assayed for MAP 4L-5. The antigens were characterized by chromatography, spectrometry and amino acid analysis. All antigens reacted in ELISA with mAb 13D9. Plain antigens and antigens coupled to P64K were used to immunize BALB/c mice. Of those variants giving immunogenic results, MAP-TT 4L-5 rendered the highest levels of specific antipeptide IgG antibodies and serum bactericidal activity. The structure MAP-TT was selected as the platform structure for further studies.
Similar structures called MAP-TT 2L-17 and MAP-TT 2L-27, with sequences 2L-17 and 2L-27 respectively, were also synthesized and simultaneously with MAP-TT 4L-5, their antigenicity and immunogenicity in mice were studied. The three structures reacted in ELISA with mAb 13D9 and inhibited binding of mAb 13D9 to purified Npr-MenB CPS. MAP-TT 4L-5 and MAP-TT 2L-27 inhibited 80% of binding, while MAP-TT 2L-17 caused approximately 50% of inhibition. The structures were immunogenic in mice. Protective SBA titers against MenB were measured in groups of mice immunized with MAP-TT 4L-5 and MAP-TT 2L-27, in group pooled sera and in more than half of the individual mice from these two groups (Table 2). The serum antibodies elicited against both antigens adsorbed to MenB CPS. Additionally, pooled sera from mice group immunized with MAP-TT 2L-27 passively protected from bacterial challenge in the infant rat model. The assessment of serum bactericidal activity of anti-MAP-TT-2L-27 sera against two other strains of serogroup B, yielded positive results. Pooled sera from mice immunized with MAP-TT 4L-5 and MAP-TT 2L-27 did not react with a homogenate of brain from neonatal BALB/c mice in an ELISA performed to evaluate the reactivity of anti-peptide antibodies with mammalian NCAM.
CONCLUSIONS
The peptide sequences 4L-5 and 2L-27 mimic the properties of a unique epitope of meningococcal serogroup B capsular polysaccharide. They induce antibodies with bactericidal activity against N. meningitidis B after mouse immunization.
The peptides identified in this study could be considered for the design of immunogenic, safe and universal vaccines against N. meningitidis serogroup B. This work has also contributed to the knowledge of properties of peptides reacting with an anti-carbohydrate antibody (14). The studies of immunization with synthetic peptides emphasized the paramount importance of the presentation form of a peptide sequence to the immune system (15).
ACKNOWLEDGEMENTS
The authors thank Dr. Harold Jennings from the National Research Council of Canada for providing mAb 13D9 as well as purified MenB CPS and HSA-conjugated Npr-MenB CPS. The authors like specially to thank the following collaborators of this work for fruitful discussions, technical or logistical support: Yaima Batista, Tania Cárdenas, Anabel Álvarez, Yanet García, Daniel Bello, Maite Ale, Clara Y Taylor, José A Silva, Alfredo Zambrano, Carmen Téllez and María de los Ángeles Fernández. We are also grateful to Mariela Vázquez and Manuel Selman-Houssein from the patents office at the CIGB, and to our colleagues from the Animal Care Unit of the CIGB for animal care and handling. Special thanks to the colleagues from the Synthetic Peptide Laboratory for peptide synthesis, purification and analysis; to those who provided us with meningococcal strains: Dr. Mark Achtman from the Environmental Research Institute, University College Cork (Cork, Ireland), Dr. Einar Rosenqvist from the National Institute of Public Health (Oslo, Norway) and the colleagues from the Finlay Institute (Havana, Cuba); and to Dr. Juan G. Arrieta for the critical reading of all literature concerning the results presented here.
REFERENCES
1. Khatami A, Pollard AJ. The epidemiology of meningococcal disease and the impact of vaccines. Expert Rev Vaccines. 2010;9:285-98.
2. Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307-26.
3. Frasch CE, Borrow R, Donnelly J. Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease. Vaccine. 2009;27 Suppl 2:B112-B116.
4. Sierra GV, Campa HC, Varcacel NM, Garcia IL, Izquierdo PL, Sotolongo PF, et al. Vaccine against group B Neisseria meningitidis: protection trial and mass vaccination results in Cuba. NIPH Ann. 1991;14:195-207.
5. Jennings HJ, Lugowski C. Immunochemistry of groups A, B, and C meningococcal polysaccharide-tetanus toxoid conjugates. J Immunol. 1981;127:1011-8.
6. Bhattacharjee AK, Jennings HJ, Kenny CP, Martin A, Smith IC. Structural determination of the sialic acid polysaccharide antigens of Neisseria meningitidis serogroups B and C with carbon 13 nuclear magnetic resonance. J Biol Chem. 1975; 250:1926-32.
7. Finne J, Leinonen M, Makela PH. Antigenic similarities between brain components and bacteria causing meningitis. Implications for vaccine development and pathogenesis. Lancet 1983;2:355-7.
8. Shin JS, Lin JS, Anderson PW, Insel RA, Nahm MH. Monoclonal antibodies specific for Neisseria meningitidis group B polysaccharide and their peptide mimotopes. Infect Immun. 2001;69:3335-42.
9. Granoff DM, Bartoloni A, Ricci S, Gallo E, Rosa D, Ravenscroft N, et al. Bactericidal monoclonal antibodies that define unique meningococcal B polysaccharide epitopes that do not cross-react with human polysialic acid. J Immunol. 1998;160:5028-36.
10. Pon RA, Lussier M, Yang QL, Jennings HJ. N-Propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. J Exp Med.1997; 185:1929-38.
11. Felici F, Castagnoli L, Musacchio A, Jappelli R, Cesareni G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J Mol Biol. 1991;222:301-10.
12. Hoess R, Brinkmann U, Handel T, Pastan I. Identification of a peptide which binds to the carbohydrate-specific monoclonal antibody B3. Gene. 1993;128:43-9.
13. Moe GR, Granoff DM. Molecular mimetics of Neisseria meningitidis serogroup B polysaccharide. Int Rev Immunol. 2001;20:201-20.
14. Menéndez T, Santiago-Vispo NF, Cruz-Leal Y, Coizeau E, Garay H, Reyes O, et al. Identification and characterization of phage-displayed peptide mimetics of Neisseria meningitidis serogroup B capsular polysaccharide. Int J Med Microbiol. 2011;301:16-25.
15. Garay H, Menendez T, Cruz-Leal Y, Coizeau E, Noda J, Morera V, et al. Study of various presentation forms for a peptide mimetic of Neisseria meningitidis serogroup B capsular polysaccharide. Bioconjug Chem. 2011;22:33-41.
Tamara Menéndez, Centro de Ingeniería Genética y Biotecnología, CIGB Ave. 31 e/ 158 y 190, Cubanacan, Playa, AP 6162, La Habana, Cuba, E-mail: tamara.menendez@cigb.edu.cu