My SciELO
Services on Demand
Article
Indicators
-
 Cited by SciELO
Cited by SciELO
Related links
-
 Similars in
SciELO
Similars in
SciELO
Share
Biotecnología Aplicada
On-line version ISSN 1027-2852
Biotecnol Apl vol.28 no.3 La Habana July-Sept. 2011
REPORT
Novel knowledge about the influence of hepatitis C virus structural proteins ratio in vaccine preparations to induce a protective immune response in a challenge model with a surrogate virus
Conocimientos novedosos sobre la influencia de la proporción entre las proteínas estructurales del virus de la hepatitis C para la inducción de una respuesta inmune protectora en un modelo de reto con virus sustituto
Alexis Musacchio, Gillian Martínez, Liz Alvarez, Ivis Guerra, Nelson Acosta, Yalena Amador, Angel Pérez, Yordanka Soria, Pedro Puentes, Santiago Dueñas
Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, La Habana, Cuba.
ABSTRACT
The incomplete definition of immunologic parameters that correlates with the protection against HCV infection has limited the development of a vaccine against this pathogen. Several studies advocate for the induction of strong cellular immune response against viral antigens, as well as neutralizing antibodies against HCV E2 envelope protein, as a success criteria. However, the influence of several antigens against each others in a protein preparation and the impact on the immunogenicity, have not been explored yet. In the present study a factorial design was used to generate several HCV vaccine preparations with the structural core, E1 and E2 proteins as antigens. An optimal antigen composition was chosen based on the lymphoproliferative response against HCV as well as protection against challenge with a surrogate virus in BALBC/c mice. The protein ratio (1:160:160), with 0.1 µg of Co.120-16.7 µg of E1.340-16.7 µg E2.680 (Co-E1-E2) was selected as the optimal composition to induce a functional immune response in mice. Additionally, strong humoral immune response against the 412-438 amino acid region from HCV E2 protein was detected when African green monkeys were immunized with Co-E1-E2. This region includes a conserved epitope, involved in T cell lymphoproliferative response against Co.120 and E2.680 proteins as well as in HCV neutralization. The results evidenced the relevance of proportion between HCV structural antigens in vaccine preparations for eliciting successful immune response.
Keywords: protective immune response, Hepatitis C, structural antigens.
INTRODUCTION
Hepatitis C is an infectious disease, caused by the hepatitis C virus. An estimated 270-300 million people worldwide are infected with hepatitis C. This virus causes chronic (long-term) infection in more than 85 percent of infected people, often leading to chronic liver disease. Pegylated interferon (PEG-IFN) combined with Ribavirin is the best currently available therapy for HCV infection. This treatment is effective only in 50% of cases, and associated side effects have been reported [1]. So, the generation of a vaccine against this pathogen would have a significant impact on control HCV infection. So far, the immunological parameters that correlate with protection from HCV infection have not been established. Studies in humans and chimpanzees suggest that a strong specific T cell response against HCV proteins is associated with spontaneous resolution of the disease [2, 3]. Evidence from human and animal models also suggest that CD4+ T cells play a critical role in controlling acute HCV infection, yet the mechanism(s) behind their failure to control chronic HCV replication are unknown [4]. On the other hand, chimpanzees immunized with HCV E1E2 proteins developed high titers of antibodies against E2, modifying the natural course of HCV infection and were protected against challenge with the homologous virus [5]. Previously, the immunogenicity of different HCV based proteins preparations have been described, however, the induction of both humoral and cellular protective immune response has not been demonstrated [6, 7]. In this work, the amount and HCV structural protein antigenic proportions, as a key factor for the induction of a protective immune response in vivo using a model of challenge with a recombinant vaccinia virus including the structural region of HCV, is reported for the first time.
RESULTS AND DISCUSSION
HCV structural proteins (Co.120, E1.340 and E2.680) obtained from recombinant microorganisms [8]; have demonstrated individually to induce a potent immune response in animal models [9]. Taking into account results from immunogenicity studies using individual proteins and the importance to induce an immune response towards several viral antigens led us to study how to combine several antigens, in term of amount and proportions, on a vaccine preparation. HCV structural proteins were combined in several ratios according to a designed factorial study (using STATGRAPHICS Plus Quality and Design software v. 5.1), and the induction of specific immune response after BALB/c mice immunization was studied. Statistical surface response analysis with data from specific cellular lymphoproliferative responses against structural HCV proteins, as well as from the challenge with a surrogate vaccinia virus comprising HCV structural region suggested that the protein ratio (1:160:160), with 0.1 µg of Co.120-16.7 µg of E1.340-16.7 µg E2.680 (Co-E1-E2) was the optimal composition to induce a functional immune response in mice (Figure 1). [10].
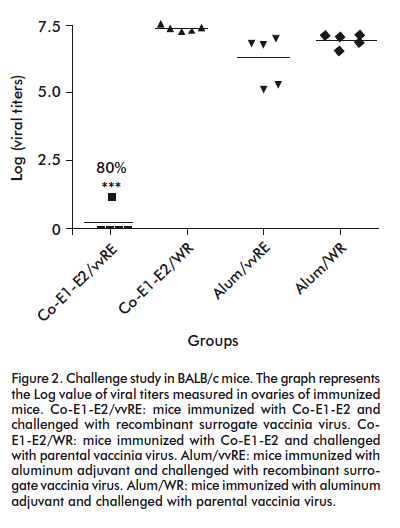
It is important to highlight the presence of HCV core protein in a lesser extent with respect to E1 and E2 envelope proteins, which enhances the immune response against E1 and E2. At the same time, this may decrease the undesirable effects related to the capsid protein in vitro demonstrated to affect cell-mediated immunity [11]. The five dose BALB/c mice immunization induced a strong specific T cell response against HCV structural proteins and 80% protection of immunized mice when challenged with recombinant surrogate vaccinia virus (Figure 2) [12].
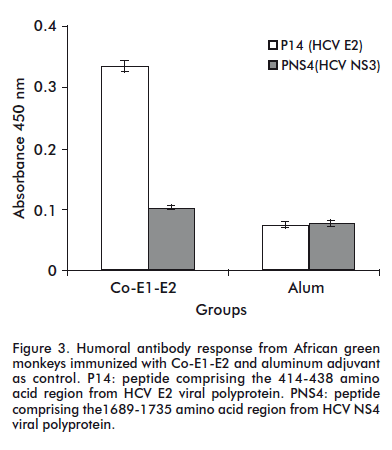
The protection against challenge with recombinant vaccinia virus has been associated with induction of T cell specific immune response against HCV antigens, involving mainly CD4+ T cells as inducers and CD8+ T cells as effectors for virus clearance [13]. The development of CD4 + and CD8 + cellular immune responses is often correlated with a benign course of hepatitis C, or with its resolution. Additionally, strong humoral immune response against the 412-438 amino acid region from HCV E2 protein was detected when African green monkeys were immunized with Co-E1-E2. This region includes a conserved epitope, involved in T cell lymphoproliferative response against Co.120 and E2.680 proteins as well as in HCV neutralization (Figure 3) [12].
The induction in chimpanzees of high antibody titers against HCV E2 protein has been shown to modify the natural course of infection and at the same time to protect against challenge with homologous virus. However, it is highly desirable to induce also neutralizing antibodies directed to relatively conserved region, making more difficult the appearance of escape mutants, frequently found due to changes mainly observed in the hypervariable region I (HVR-I). The region comprising aa 412-419, located immediately downstream of the HVR-I is a target for neutralizing antibodies and is more conserved because is critical for CD81 binding [14]. Interestingly, strong humoral immune response against the 412-438 amino acid region from HCV E2 protein was detected when African green monkeys were immunized with Co-E1-E2 (Figure 3). This response might be additionally supported by the presence of some conserved epitopes, involved in T cell lymphoproliferative response against Co.120 and E2.680 proteins in this protein preparation [12].
The presence of particulate and aggregate antigens (Co.120 and E2.680) in the Co-E1-E2 formulation is expected to provide an important contribution in terms of capture, processing and presentation by antigen-presenting cells in order to effectively activate an immune response against HCV structural antigens [12]. In this scenario, antigen to-antigen ratio in the preparation is logically critical for accurate interaction between recombinant HCV structural proteins and should have implications on the immunogenicity.
These results demonstrate for the first time that the ratio of HCV proteins is a key factor to induce an effective immune response, indicating an immunogenic hierarchy among these antigens, and therefore constitute an important element to consider for the design of recombinant vaccines against HCV.
REFERENCES
1. Caruntu FA, Benea L. Acute hepatitis C virus infection: Diagnosis, pathogenesis, treatment. J Gastrointestin Liver Dis. 2006; 15:249-56.
2. Veldt BJ, Saracco G, Boyer N, Cammà C, Bellobuono A, Hopf U, et al. Long term clinical outcome of chronic hepatitis C patients with sustained virological response to interferon monotherapy. Gut. 2004; 53:1504-8.
3. Houghton M, Abrignani S. Prospects for a vaccine against the hepatitis C virus. Nature. 2005;436:961-6.
4. Gerlach JT, Diepolder HM, Jung MC, Gruener NH, Schraut WW, Zachoval R, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 1999; 117:933-41.
5. Houghton M, Choo QL, Chien D, Kuo G, Weiner A, Coates S, et al. Development of an HCV vaccine. In: Rizzetto M, Purcell RH, Gerin JL, Verme G, editors. Viral hepatitis and liver diseases. Turín: Minerva Medica; 1997. p. 656-9.
6. Li P, Wan Q, Feng Y, Liu M, Wu J, Chen X, et al. Engineering of N-glycosylation of hepatitis C virus envelope protein E2 enhances T cell responses for DNA immunization. Vaccine. 2007;25:1544-51.
7. Puig M, Major ME, Mihalik K, Feinstone SM. Immunization of chimpanzees with an envelope protein-based vaccine enhances specific humoral and cellular immune responses that delay hepatitis C virus infection. Vaccine. 2004;22:991-1000.
8. Martinez-Donato G, Acosta-Rivero N, Morales-Grillo J, Musacchio A, Vina A, Alvarez C, et al. Expression and processing of hepatitis C virus structural proteins in Pichia pastoris yeast. Biochem Biophys Res Commun. 2006;342:625-31.
9. Acosta-Rivero N, Poutou J, Alvarez-Lajonchere L, Guerra I, Aguilera Y, Musacchio A, et al. Recombinant in vitro assembled hepatitis C virus core particles induce strong specific immunity enhanced by formulation with an oil-based adjuvant. Biol Res. 2009;42:41-56.
10. Musacchio A, Duenas-Carrera S, Alvarez-Lajonchere L, Acosta-Rivero N, Martinez-Donato G, Guirola M, et al., inventors; Center for Genetic Engineering and Biotechnology, asignee. Vaccine composition against hepatitis c virus. International patent WO2006024240. 2006 Mar 9.
11. Krishnadas DK, Ahn JS, Han J, Kumar R, Agrawal B. Immunomodulation by hepatitis C virus-derived proteins: targeting human dendritic cells by multiple mechanisms. Int Immunol. 2010;22:491-502.
12. Martínez-Donato G, Musacchio A, Alvarez-Lajonchere L, Acosta-Rivero N, Amador Y, Guerra I, et al. Ratio of HCV structural antigens in protein-based vaccine formulations is critical for functional immune response induction. Biotechnol Appl Biochem. 2010;56:111-8.
13. Murata K, Lechmann M, Qiao M, Gunji T, Alter HJ and Liang TJ. Immunization with hepatitis C virus-like particles protects mice from recombinant hepatitis C virus-vaccinia infection. Proc Natl Acad Sci USA. 2003;100:6753-8.
14. Zhang P, Wu CG, Mihalik K, Virata-Theimer ML, Yu MY, Alter HJ, et al. Hepatitis C virus epitope-specific neutralizing antibodies in Igs prepared from human plasma. Proc Natl Acad Sci USA. 2007;104:8449-54.
Alexis Musacchio. Centro de Ingeniería Genética y Biotecnología, CIGB. Ave. 31 e/ 158 y 190, Cubanacán, Playa, La Habana, Cuba. E-mail: alexis.musacchio@cigb.edu.cu .